SUGAR ALLYLTINS: PREPARATION AND APPLICATION IN ORGANIC SYNTHESIS
Sławomir Jarosz
ul. Kasprzaka 44/52, 01-224 Warszawa, POLAND
e-mail: sljar@icho.edu.pl
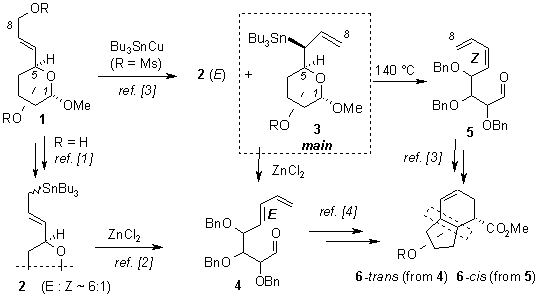
In the past few years we elaborated a convenient route to sugar allyltin derivatives 2 from the corresponding allylic alcohols 1 [1]. These derivatives undergo a controlled rearrangement to dienoaldehydes 4 upon treatment with zinc chloride [2].

Reaction of sugar allylic mesylates with ‘Bu3SnCu’ gives predominantly secondary sugar allyltin 3 as the only stereoisomer [2,3]. This compound decomposes to E-diene 4 upon treatment with ZnCl2, but its thermal decomposition at 140 °C provides the opposite Z-isomer 5. Both dienes 4 and 5 were used for the preparation of bicyclo[4.3.0]nonenes differing in configuration between the five- and six-membered rings.
References:
[1]. Kozłowska, E.; Jarosz, S. J. Carbohydr. Chem. 1994, 13, 889–898.
[2]. Jarosz, S. Tetrahedron, 1997, 53, 10765–10774.
[3]. Jarosz, S.; Kozłowska, E.; Jeżewski, A. Tetrahedron 1997, 53, 10775–10782.
[4]. Jarosz, S.; Szewczyk, K. Tetrahedron Lett, 2001, 42, 3021–3024