DOUBLE ASYMMETRIC INDUCTION IN 1,3-DIPOLAR CYCLOADDITION OF NITRONES TO 2,3-UNSATURATED SUGAR 1,5-LACTONES
Konrad Paśniczek, Dariusz Socha, Margarita Jurczak and Marek Chmielewski
e-mail: chmiel@icho.edu.pl
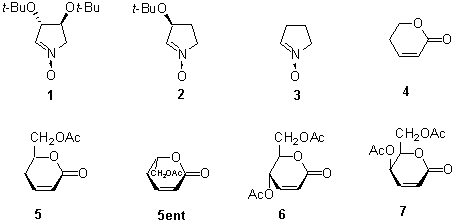
1,3-Dipolar cycloaddition of nitrones 1-3 to the α,β-unsaturated δ-lactones: non-chiral 4, D-glycero 5, DL-glycero 5/5ent, D-erythro 6, and D-threo 7 provides an interesting example of a double asymmetric induction. In all cases only the exo approach of reactants was observed. The high preference of anti addition of the nitrones to the terminal acetoxy-methyl group in the lactones 5-7 is consistent with the previous observations. The 3-t-butoxy group of the nitrone plays a similar role. In the case of the mismatched pairs, the location of the 4-O-acetoxy substituent in the lactone and 4-t-butoxy in the nitrone become decisive for the direction of addition.
