MOdern aspects of sugar chemistry:
GLYCIDIC SCAFFOLDS
For the production of bioactive compounds
Department of Biotechnology and Bioscience, University of Milano Bicocca,
P.zza della Scienza, 2; 20126 Milano (Italy).
Most cell-cell and cell-pathogen interactions and signalling are mediated by the glycidic part of cell-wall glycolipids and glycoproteins. Essential elements to be taken into consideration in these recognition phenomena are not only some functional groups (pharmacophores) of the natural molecule, but also the conformational rigidity of the glycidic backbone that act as a scaffold properly orienting those groups in the space. Inspired by the role of carbohydrates as natural scaffolds, interesting and innovative studies have been performed in order to use conformationally constrained carbohydrate derivatives for the production of bioactive compounds with specific structural requirements. Furthermore, the wide diversity of carbohydrates in terms of chirality, and the possibility to derivatise in different ways their functional groups, has been exploited for the generation of libraries in the search of new pharmaceuticals.
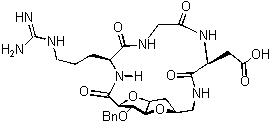
An important element to be taken into consideration for the generation of carbohydrate derived scaffolds is the possibility to link different monomers in a chemoselective way in order to obtain oligosaccharide analogues avoiding the tedious and time consuming protection/deprotection steps. Recent efforts in the development of conformationally constrained glycidic scaffolds presenting an amino and a carboxylic function (Sugar AminoAcids, SAA) will be presented. These compounds are interesting monomers that can be elongated by traditional peptide synthesis, not only allowing the production of new glycomimetics, but also inducing peptide loops in peptidomimetics (see figure 1).


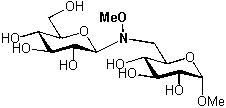
Figure 1 Figure 2
In order to simplify the synthesis of oligomers, a chemoselective ligation procedure, involving the carbonyl function of the anomeric centre of a natural unprotected sugar, and the unnatural methoxyamino function inserted in a second sugar, has been developed (see figure 2).