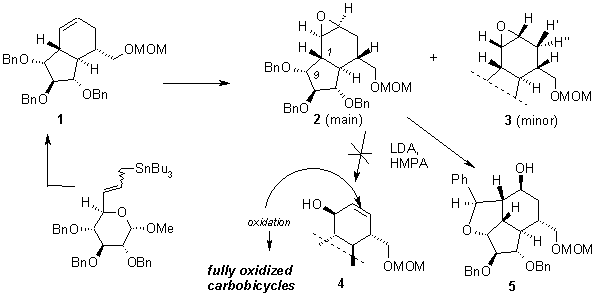
Epoxidation of he bicyclic precursor 1 (obtained by a standard methodology from the correspon-ding sugar allyltin1,2) was accomplished with MCPBA to give both epoxides 2 and 3.2 Base catalyzed isomerization of these epoxides should open a convenient route to allylic alcohols (e.g. 4), which could be precursors of highly oxygenated carbobicyclic derivatives.

Isomerization of the model 2,3-epoxy-7,8,9-tri-O-benzyl-5-substituted bicyclo[4.3.0]nonane derivative 2 was accomplished with LDA under rather harsh conditions (LDA, HMPA, 80 °C, 2 days). However, the product was not the expected allylic alcohol 4, but the tricyclic derivative 5, which resulted from the ring opening of the oxirane by the anion generated from the benzyl group at C9, as was proved by advanced NMR experiments.
1. S. Jarosz, E. Kozłowska, A. Jeżewski, Tetrahedron, 53, 1997, 10775.
2. S. Jarosz, B. Boryczko, P. Cmoch, to be published