SOLID-PHASE SYNTHESIS OF CLAVAMS AND OXACEPHAMS
Marek Chmielewski, Maciej Cierpucha, Bartłomiej Furman, Barbara Grzeszczyk, Zbigniew Kałuża,
Robert Łysek
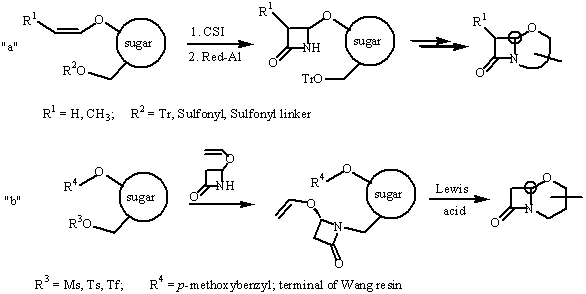
Two complementary methods leading to clavams and 5-oxacephams (Scheme) were employed under solid-phase conditions. In the route “a” the sugar vinyl ether is bound to the polymer via sulfonyl linker, whereas in the route “b” terminal of the Wang resin protects the oxygen atom which plays subsequently a role of the nucleophile.
Scheme

In the case of 5-oxacephams routes “a” and “b” provide alternative configuration at the bridgehead carbon atom (C-6). Clavams can be obtained only via the [2+2]cycloaddition method. In both methods the cyclization/cleavage methodology is used to construct the bicyclic skeleton.