
Automated Purification, Immobilization and Reaction Control of Enzymes in Flow-through Reactors
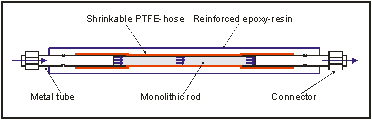
Immobilization of recombinant enzymes using the well established His6-tag-technology1 is the basic principle of the presented novel flow-through reactor. Starting with the efficient Passflow reactor2, a chemically functionalized highly porous polymer/glass-monolith, the synthesis of a Ni-NTA matrix and the optimization of the polymer backbone is achieved.



Scheme 1: Inner view of a Passflow reactor
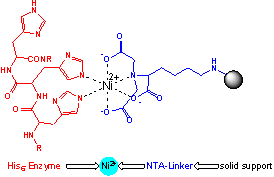 These
reactors can be automatically purged using different solutions. This opens the
possibility to purify and immobilise enzymes within minutes and subsequently
perform enzymatic transformations using these immobilized catalysts. Afterwards,
the reactor can be easily purged and regenerated for the next use.
These
reactors can be automatically purged using different solutions. This opens the
possibility to purify and immobilise enzymes within minutes and subsequently
perform enzymatic transformations using these immobilized catalysts. Afterwards,
the reactor can be easily purged and regenerated for the next use.
1. Augé, C, Malleron, A., Tahrat, H., Marc, A., Goergen, J.-J., Cerutti, M., Steelant, W.-F., Delannoy, P. and Lubineau, A. (2000). Outstanding stability of immobilized recombinant a(1®3/4)-fucosyltrans-ferases exploited in the synthesis of Lewis a and Lewis x trisaccharides. Chem. Commun. 2017-2018.
2. Kirschning, A., Altwicker, C., Dräger, G., Harders, J., Hoffmann, N., Hoffmann, U., Schönfeld, H., Solodenko, W. and Kunz, U. (2001). PASSflow syntheses using functionalized monolithic polmer/glass composites in flow-through microreactors. Angew. Chem. Int. Ed., 40, 3995-3998.