Application of Transition
Metal Acylates for Determination of the Absolute Configuration of vic-Amino
Alcohols
by Circular Dichroism
Spectroscopy
Jadwiga
Frelek
Institute of
Organic Chemistry, Polish Academy of Sciences, Warsaw, Poland
Amino alcohols represent an important group of organic
compounds due to their significant biological activity and wide application in
synthesis. For those reasons the determination of their absolute configuration
is very important. To enable the circular dichroic studies on amino alcohols
that are transparent in an
accessible spectral range,
a suitable chromophoric system needs
to be introduced into the molecule. One possible solution is based on the
methodology that consists of making chiral complexes in situ with achiral
transition metal complex displaying adequate absorption characteristics.
|
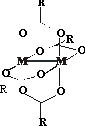 |
The aim of this work is to establish
whether the transition metal acylates of general formula [M2(O2CR3)4]
with M = Mo, Rh can be used as
appropriate auxiliary chromophores for reliable, rapid and effective
determination of the absolute
configuration of vic-amino alcohols. As model compounds a
variety of amino alcohols of both ephedrine and adrenaline types has been
chosen. During the present study the dependence of the |
CD spectra
on the ligand to stock complex ratio, on a solvent used as well as on time will
be tested. For this purpose, the electronic absorption spectroscopy will be used
in addition to the circular dichroism. In the case of dirhodium tetraacetate to
explain the possible mechanism of complexation of amino alcohols to the Rh2-core,
i.e., chelating or bridged type of bidentate ligation or unidentate
ligation, the 1H and 13C NMR spectroscopy will be also
applied.
The resulting CD spectra are suitable for the assignment of absolute
configuration, since the observed signs of Cotton effects arising within the
d-d
absorption bands of the metal cluster depend solely upon the chirality of the
amino alcohol ligand. Therefore, a straightforward and versatile method for the
determination of the absolute configuration of vic-amino alcohols can be
proposed. In the case of dimolybdenum tetraacetate an empirically based rule
correlating a positive/negative helicity expressed by the O-C-C-N
torsional angle with the sign of Cotton effects occurring in the 400 – 260 nm
spectral range has been formulated. In the case of dirhodium tetaracetate a
correlation between structure of an amino alcohol and sign of the CD band
arising around 600 nm has been found.
This work is
supported by the CSR (Committee of Scientific Research) grant No. 7 T0 9A 004
21.
