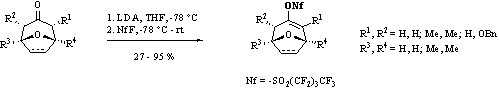
Nonaflates of Oxabicyclo[3.2.1]oct-6-en-3-ones and their Palladium Catalysed Coupling Reactions
Jens Högermeier, Hans-Ulrich Reissig
Institut für Chemie, Freie Universität Berlin, Germany
Perfluoroalkylsulfonylated reagents are of high interest in metal catalyzed coupling reactions. Recently, nonafluorobutanesulfonates1 have been used in our group as coupling partners in different Pd-coupling reactions2 and have shown to be very useful tools. To investigate the scope and limitations we have focussed our interest3,4 towards the application of nonaflation of oxabicyclo[3.2.1]octene derivatives and related systems. Starting from the carbonyl compounds we directly synthesized the corresponding nonaflates by deprotonation and reaction with the cheap and easy-to-handle nonafluorobutanesulfonylfluoride (NfF) as nonaflating reagent.
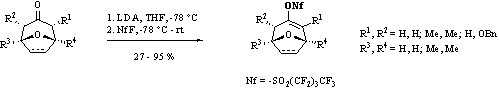
With the nonaflates in hand we tested their application to coupling reactions focusing on their use in Heck-, Sonogashira- and Suzuki-couplings. We were able to establish coupling protocols receiving the products in good yields. This contributes to the synthetic diversity of oxabicyclic[3.2.1]octadienones.5
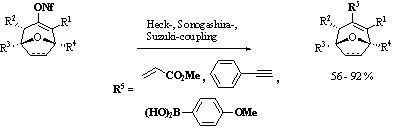
Furthermore we use these couplings to establish a route to synthesize polycyclic compounds via a Heck-coupling / Diels-Alder one pot reaction sequence. The diene for the cycloaddition can be synthesized by a Heck-coupling followed by a [4+2]-cycloaddition using for example N‑phenylmaleimide as dienophile resulting in stereoselective formation of tetracyclic compounds.
1. I. M. Lyapkalo, M. Webel, H.-U. Reissig, Eur. J. Org. Chem. 2002, 1015-1025;
2. I. M. Lyapkalo, M. Webel, H.-U. Reissig, Eur. J. Org. Chem. 2002, 3646-3658;
3. I. M. Lyapkalo, M. Webel, H. U. Reissig, Eur. J. Org. Chem. 2001, 4189-4194;
4. I. M. Lyapkalo, M. Webel, H.-U. Reissig, Synlett 2001, 1293-1295;
5. H. M. R. Hoffmann, I. V. Hartung, Angew. Chem. 2004, 116, 1968-1984; Angew. Chem. Int. Ed. 2004, 43, 1934-1949.