Synthesis of (-)-Anisomycin by Reaction of Lithiated Alkoxyallenes with Imines
Silvia Kaden, Mirjam Kratzert, Hans-Ulrich Reissig
Institut für Chemie, Freie Universität Berlin, Germany
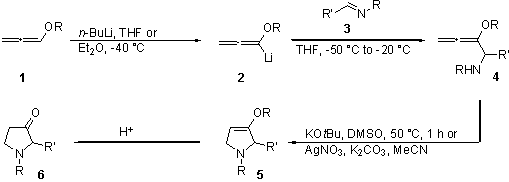
Alkoxyallenes 1 are valuable building blocks for the synthesis of heterocyclic structures.1 Deprotonation of alkoxyallenes 1 with n-butyllithium leads to the lithiated species 2, which can react with imines 3 to the primary adducts 4. These adducts 4 give dihydropyrrole derivatives 5 in good yields by cyclization using silver nitrate or potassium-tert-butoxide catalysis.2 By hydrolysis pyrrolidinones 6 are obtained from the intermediate dihydropyrroles.

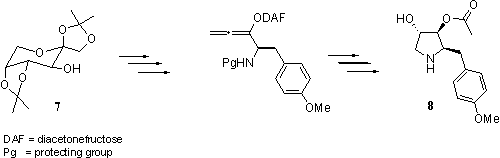
This strategy was applied to the total synthesis of the antibiotic (-)-anisomycin 8 starting from diacetonefructose 7. (‑)‑Anisomycin 8 specifically blocks peptide bond formation on eukaryotic ribosomes and therefore exhibits selective action against protozoa and several strains of fungi. It also shows high in vitro antitumor activity.3,4 The chiral centre at C-2 was generated by an allene containing a chiral auxiliary. The other chiral centres were obtained by substrate control.
1. Reviews: a) R. Zimmer, Synthesis 1993, 165. b) R. Zimmer, F. A. Khan, J. Prakt. Chem. 1996, 338, 92. c) H.-U. Reissig et al., J. Heterocyclic Chem. 2000, 37, 597.
2. M. Okala Amombo, A. Hausherr, H.-U. Reissig, Synlett 1999, 1871.
3. T. Kameyama, Y. Hosoya, H. Naganawa, Y. Okami, T. Takeuchi, J. Antibiotics 1993, 46, 1300.
4. a) A. Jimenez, D. Vazquez, Antibiotics, Hahn, F. E.; Hrsg.; Springer Verlag: Berlin, 1979, pp 1-19. b) J. E. Lynch, A. R. English, H. Banck, H. Deligianis, Antibiot. Chemotherapy 1954, 4, 844. c) A. P. Grollman, J. Biol. Chem. 1967, 242, 3226.