Efficient Synthesis of Natural Products and Analogues by Metal-Mediated Transformations
Armin de Meijere, Hans
Wolf Sünnemann, Farina Brackmann, Oleg Larionov,
Boris Zlatopolskiy
Institut für Organische und
Biomolekulare Chemie, Georg-August-Universität Göttingen, Germany
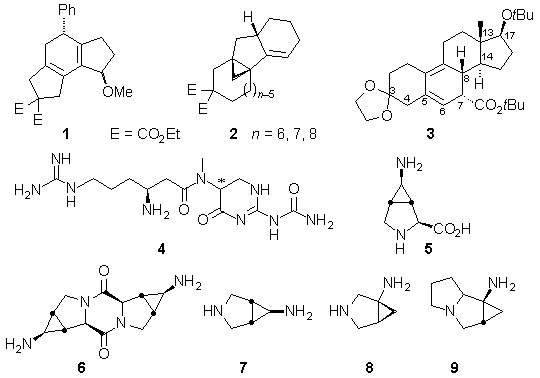
Newly developed palladium-catalyzed sequential reactions offer elegant accesses to tri- and tetracyclic skeletons like 1 and 2 from simple precursors. In this context, our recently published1 new construction of steroidal molecules like 3 will be discussed.

Our titanium-mediated reductive cyclopropanation of N,N-dialkylcarboxamides to yield N,N-dialkylcyclopropylamines2 has been exploited to prepare various interesting cyclopropyl-containing amino acids like analogues of the b-amino acid moiety in the important antiinfectant TAN 1057 A/B 4 as well as the 3,4-(aminomethano)proline 5 as a new hairpin inducing motif in peptidomimetics. The diketopiperazine 6 derived from 5 as well as the rigid bicyclic and tricyclic diamines 7–9 offer themselves as scaftolds for various biophoric groups.
1. H. W. Sünnemann, A. de Meijere, Angew. Chem. 2004, 116, 913–915; Angew. Chem. Int. Ed. 2004, 43, 895–897.
2. (a) A. de Meijere, S. I. Kozhushkov,A. I. Savchenko, in Titanium and Zirconium in Organic Synthesis (Ed.: I. Marek), Wiley-VCH, Weinheim, 2002, p. 390–434. (b) A. de Meijere, S. I. Kozhushkov,A. I. Savchenko, J. Organomet. Chem. 2004, in press.