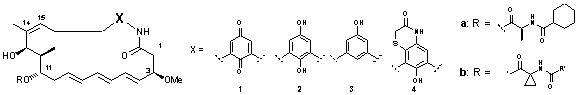
Chemistry and Biology of Ansamycin Antibiotics
Institut für Organische Chemie, Universität Hannover, Germany
The ansamycins comprise a growing class of complex macrolactam antibiotics from microbial sources. They are characterized by a cyclic structure in which an aliphatic ansa chain forms a bridge between two non-adjacent positions of a cyclic p-system. Many of them exhibit antibacterial, antifungal or antitumor activity. A subgroup which has seen increasing interest lately are benzenic ansamycins with a 17 carbons and one nitrogen atom ansa chain.
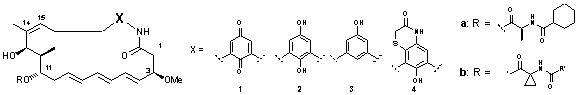
Scheme 1: Structures of Ansamycin Antibiotics
In 1981, one of the first examples, the ansatrienines 1a and 2a, were isolated from the fermentation broth of Streptomyces collinus by Zähner, Zeeck and coworkers1,2. The absolute configuration was determined in 19903 and proved in 1996 by the first total synthesis4. We developed a synthetic strategy for this class of natural products5 which utilizes three building blocks A - C as main components. In the first phase of the highly convergent synthesis fragments B and C were planned to be coupled prior to introduction of fragment A. This strategy would permit the synthetic access to the whole family of benzenic ansamycins 1- 4 and unnatural analogues. In our recent works, we focus on the total synthesis of Ansatrienin A (1a);the retrosynthetic attempt can be seen in the scheme below.

Scheme 2: Retrosynthetic Analysis
1. Zähner, Lazar, Damberg, and Zeeck, J. Antibiot., 1983, 36, 187-189. Zeeck, Damberg, and Russ, Tetrahedron Lett., 1982, 23, 59-62
2. Sasaki, Furihata, Seto, and Otake, J. Antibiot., 1982, 35, 1474-1479.
3. a) A. B. Smith III, J. L. Wood, W. Wong, A. E. Gould, C. J. Rizzo, J. Am. Chem. Soc., 1990, 112, 7425-7426; b) A. B. Smith, III, J. L. Wood, Tetrahedron Lett., 1991, 32, 841-842.
4. A. B. Smith, III, J. L. Wood, W. Wong, A. E. Gould, C. J. Rizzo, J. Barbosa, K. Komiyama, S. Omura, J. Am. Chem. Soc., 1996, 118, 8308-8315.
5. Schöning, Hayashi, Powell, and Kirschning, Tetrahedron Asymm., 1999, 10, 817-820