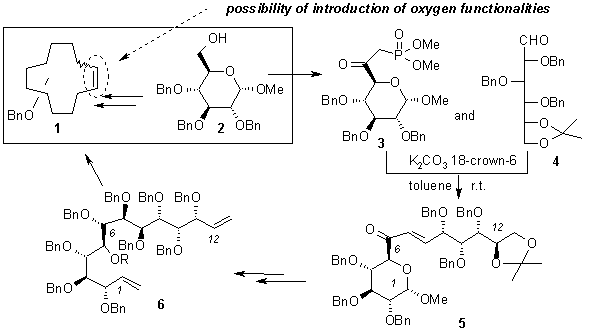
The concise approach towards the enantiomerically pure, highly oxidized macrocyclic derivatives with the large rings (e.g. 1) will be presented.

Starting from the same substrate 2, the phosphonate 3 and the aldehyde 4 were prepared by standard methods. Coupling of these two precursors to enone 5 was conveniently performed under very mild PTC conditions. Stereoselective reduction (at-C6) of 5 with zinc borohydride, followed by further rather standard synthetic steps afforded the olefin 6. The key step in the synthesis of macrocycles 1 will consist of the well documented RCM reaction.1
1. A. Fürstner, Angew. Chem., Int. Ed. Engl. 2000, 39, 3013.