VINYLOXY-ALKOXIDES
NEW DIASTEREOSELECTIVE C-C-COUPLING REACTIONS OF CARBOHYDRATE VINYL ETHERS WITH CARBONYL COMPOUNDS
Dörthe Vortmeyer
Organisch-Chemisches Institut, Westfalische Wilhelms - Universität, Corrensstraße 40, 48149 Münster, Germany
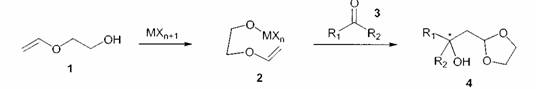
Vinyloxy-ethanol 1 and derivatives are qualified reagents for carbon-carbon-coupling reactions[1,2,3]. The reaction occurs via formation of the intermediate 2 followed by attack of the carbonyl component 3 to get the β-hydroxy-1,3-dioxolanes 4.

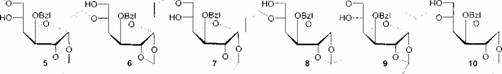
To apply this new type of aldol reaction in carbohydrate chemistry the vinyl ethers 5 and 6 and the (E7Z)-1-propenyl ethers 7, 8, 9 and 10 are synthesized [4]. Each of them bearing a free hydroxyl group in a-position to the ether moiety.
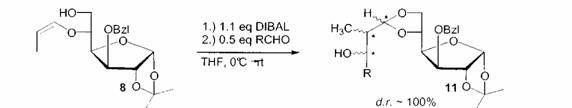
In the following step the vinyl ethers are treated with a metall reagent and a carbonyl compound to give C-C-coupling products like 11. The reaction allows to control up to three new chiral centers in only one step with good to excellent diastereoselectivity [4].
References
[1] M. Schmeichel, H. Redlich, Synthesis 1996, 1002.
[2] P. Maier, H. Redlich, Synlett 2000, 257.
[3] P. Maier, Ph. D. Thesis, Universita't Munster 2003.
[4] D. Vortmeyer, Ph. D. Thesis, Universitat Munster 2004.