
The goal of our group
NOVEL STRATEGIES FOR GREENER ORGANIC SYNTHESIS
We develop novel strategies for efficient organic synthesis in accordance with the principles of circular chemistry. It is the goal of our group to face up to our self-inflicted problems (pollution) and provide means to create methods for both generating greener production of valuable chemicals and degradation of organic compounds. Although these two ideas seem contradictory, a chemist only sees them as complex organic reactions that require a colorful imagination and a steadfast determination to succeed. How can we stand toe to toe with such a mammoth problem?It is the goal of our lab to create 'greener' methodologies for the production of valuable chemicals.
To address current sustainability issues of the chemical industry, we need to develop alternative technologies to eliminate environmentally damaging chemicals and by-products. This can only be achieved by taking inspiration from the Nature as 'what works' is based on its 3.8 billion years of evolution. Nature constructs and decomposes complex molecules with the help of enzymes as catalysts and light as the ultimate energy source. However, despite fundamental benefits of enzymes, their industrial application can be limited by their availability, instability towards certain conditions, cost etc.
Our lab develops catalytic methods that mimic efficiency characteristic for enzymes by combining the robust nature of simple nature-derived catalysts with light as the source of energy.
In particular, we have already developed multiple chemical transformations based on photochemical reactions of porphyrins and vitamin B12 as catalysts.
For more information see our publications page
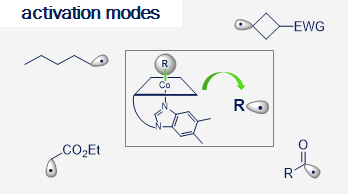
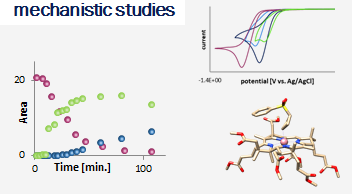
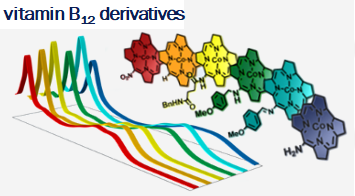
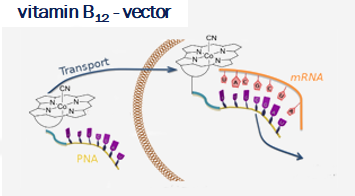
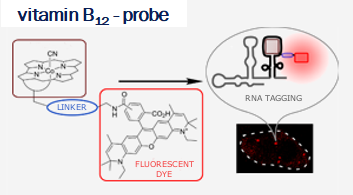
Vitamin B12 (cobalamin) is an essential nutrient for mammals and certain bacteria. As an exogenous compound, cobalamin reaches mammalian cells via a system of transport proteins and this fact makes it an attractive candidate for the delivery of cargoes into cells. Development of conjugates requires suitably designed building blocks and vitamin B12 possesses a number functional groups susceptible to chemical modifications, however complex nature of cobalamin makes them extremely challenging.
Our lab develops new methodologies allowing for the preparation of cobalamin derivatives which can be used for the synthesis of vitamin B12 conjugates with biologically relevant molecules.
For more information see our publications page
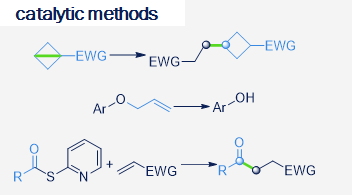
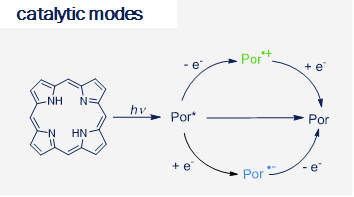
Our research focuses on the development of new synthetically useful methods for transformation of organic molecules that uses visible light as primary energy source. Employing visible light to drive organic transformations, aside from potential ability to harvest the tremendous amounts of energy provided by the sun, allows to overcome high-activation-energy barriers in mild conditions, favoring high selectivity of the transformations, in special cases leading to products which are not available by other means. As most of organic molecules don't absorb visible light, in most cases the presence of the photocatalyst is required. Our research also covers development of new photocatalytic systems and we are particularly interested in utilizing organic dyes as photocatalysts.
For more information see our publications page
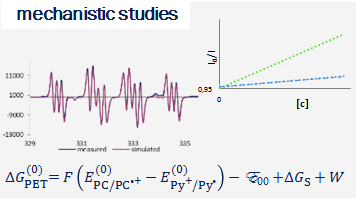
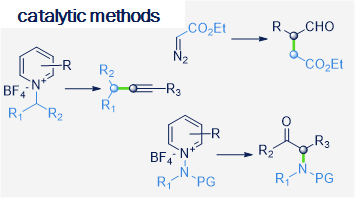
The research interests of the group focus on the development of novel synthetic methods, which utilize the energy of light to fuel chemical reactions. Our studies involve the use of aqueous, microheterogenous solutions as efficient photocatalytic systems of unique properties. In particular, we explore methods for converting stable and readily accessible starting materials through selective activation of strong chemical bonds.
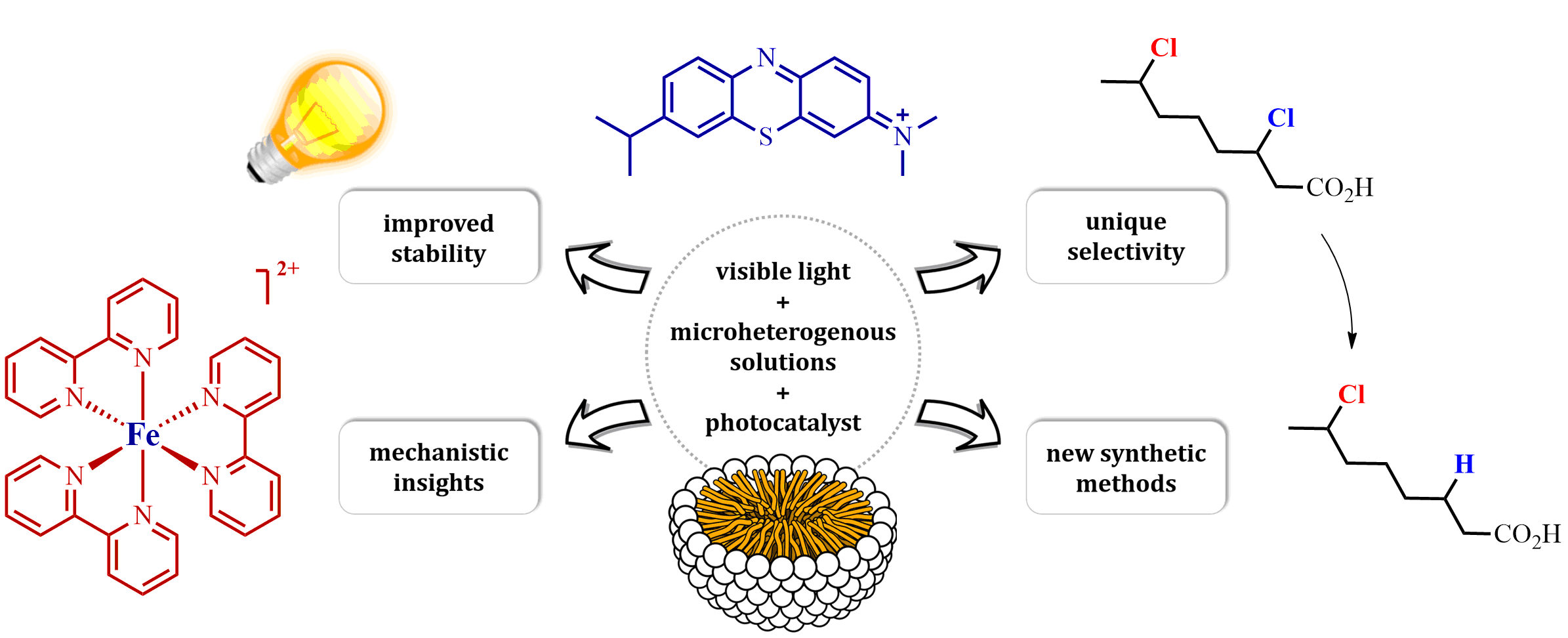
For more information see M. Giedyk's publications page


