Arkadiusz Listkowski and Sławomir Jarosz
ul. Kasprzaka 44/52, 01-224 Warszawa, POLAND
e-mail: listek@icho.edu.pl
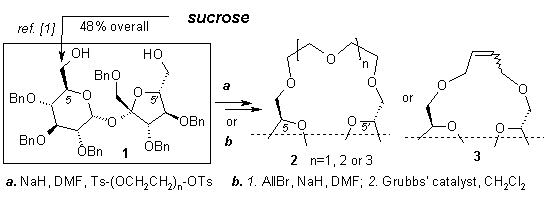
Recently we elaborated a convenient route to 1’,2,3,3’,4,4’-hexa-O-benzylsucrose 1 from free sucrose [1]. This compound was applied for the preparation of macrocycles containing the sucrose bacbone.

Reaction of 1 with several ditosylates under standard conditions used for construction of such macrocycles afforded chiral crown ether analogs 2. The benzyl protecting groups were conveniently removed by simple hydrogenolysis. On the other hand, diol 1 was converted into the diallyl ether, which under the metathesis conditions gave a mixture of the cis and trans cyclic olefins 3 in good yield. All newly formed macrocycles and their deprotected derivatives will be used for enantioselective comlexation of chiral amines.
References:
[1]. Jarosz, S.; Listkowski, A. J. Carbohydr. Chem., 2003, 22, in press.