In the 1950ís pseudouridine, a carbon-linked ribonucleoside, was discovered as a component of ribonucleic acid. A decade later several other C-linked nucleosides, with antitumour and antibiotic properties, were isolated from microbial sources.
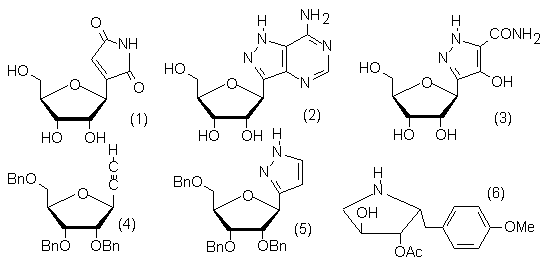
When I moved to Heriot-Watt University, Edinburgh in 1969 we embarked on the synthesis of the naturally occurring C-nucleosides showdomycin (1), formycin (2) and pyrazofurin (3). The methods were chosen so that related structures could be obtained and their biological activity determined. The ribofuranosylethyne (4) and ribofuranosyl pyrazole (5) were our immediate targets. I shall discuss their synthesis and their conversion into the target C-nucleosides, together with D-arabino and D-xylo analogues of the pyrazole (5).

We were attracted by the possibility of using similar chemistry to synthesise the antiprotozoal antibiotic anisomycin (6) using D-erythrose or D-ribose as an enantiopure starting material. This synthesis will also be discussed together with the synthesis of other classes of hydroxylated pyrrolidines related to glycosidase inhibitors.
My main collaborators in this work were Dr Alan Edgar and Dr Richard Wightman. It was carried out in the Department of Chemistry, Heriot-Watt University, Edinburgh.