a-Hydroxyketones in Asymmetric Synthesis: From Chiral Auxiliaries to Achiral Templates For Catalytic Enantioselective Processes
Claudio Palomo Nicolau
Departamento de Química Orgánica I, Facultad de Química
Universidad del País Vasco, Apdo. 1072, 20080 San Sebastián, Spain
E-mail: qoppanicc.ehu.es
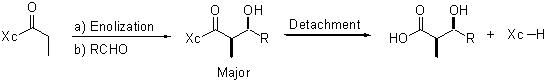
Control of the stereochemistry in a chemical transformation is a key issue in modern organic chemistry, which has led to the preeminence of asymmetric synthesis. One option for controlling stereochemistry and thus for producing one of the stereoisomers over the other possible ones in a predictable fashion, is the use of a stoichiometric chiral auxiliary which is covalently attached to the prochiral substrate before chirality relay is performed. The auxiliary is removed for reuse once the new stereogenic center is built with the desired configuration. A typical example is the aldol reaction of acyl derivatives using a-amino acid-derived oxazolidinones and the camphorsultam pioneered by Evans and Oppolzer, respectively, as chiral auxiliaries (Xc).

A more advanced and atom economic option for controlling stereochemistry relies on the use of a chiral catalyst, typically a transition metal complex, which is reversibly bound to the prochiral substrate in the catalytic cycle. Substrate-chiral catalyst complexation promotes substrate activation and asymmetric induction, while reversibility is responsible for product liberation and catalyst recycling. A typical reversible complexation platform is provided by the carbonyl group-metal interactions in either monodentate or bidentate patterns.
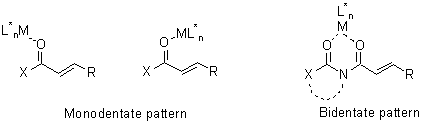
In this lecture it will be demonstrated that a diversity of reactions, i.e. aldol, Mannich and Diels-Alder reactions, can be carried out on a-hydroxy ketones asymmetrically, in both chiral auxiliary-mediated diastereoselective way and chiral catalyst-promoted enantioselective way. The understanding of the stereochemical outcome of the reactions and the key features for the design of auxiliaries and templates will be presented as well.
