Oxy
Anion Accelerated Reactions of
Arene Tricarbonylchromium Complexes
Holger Butenschön
Institut für Organische Chemie, Universität Hannover, Germany
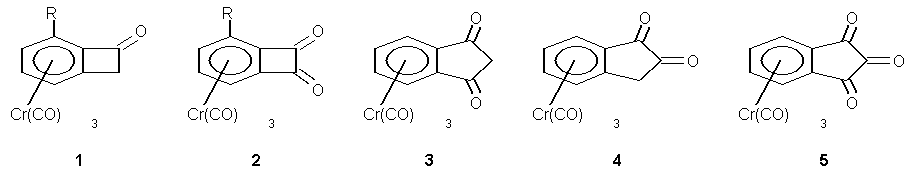
The lecture will summarize our chemistry starting from tricarbonylchromium complexes 1and 2 (R = H, OMe) of benzocyclobutenone and benzocyclobutenedione. While 1 is a basis for ring opening / cycloaddition sequences and acyl anion driven ring expansion reactions,1 2 undergoes syn-diadditions of nucleophiles. When alkenyl metal reagents are used, the diaddition is followed by a dianionic oxy-Cope rearrangement and an intramolecular aldol addition at –78 °C with complete diastereoselctivity, finally leading to highly substituted benz-anellated di-, tri- or tetraquinanes.2 This chemistry involves the formation of eight membered rings with two different enolate moieties present, which are fully discriminated in the following regio an diastereoselective intramolecular aldol addition. Recent developments in this chemistry include applications of the dianionic oxy-Cope rearrangement to metal free benzil derivatives as substrates,3 the formation of complicated heterocycles by dianionic oxy-Cope rearrangements4 as well as the formal total synthesis of enantiomerically pure isochroanones.5 In addition to these reactions some unusual structural properties of 1 (R = H, OMe, CF3) and 2 (R = H, OMe) will be reported.6 Further, the syntheses and some reactions of higher homologues such as 3-5 and related ligand systems will be presented.7
1. H. Ziehe, R. Wartchow, H. Butenschön, Eur. J. Org. Chem. 1999, 823-835.
2. H. Butenschön, Synlett 1999, 680-691.
3. C. Clausen, R. Wartchow, H. Butenschön, Eur. J. Org. Chem. 2001, 93-113.
4. B. Voigt, R. Wartchow, H. Butenschön, Eur. J. Org. Chem. 2001, 2519-2527.
5. M. Schnebel, I. Weidner, R. Wartchow, H. Butenschön, Eur. J. Org. Chem. 2003, 4363-4372.
6. K. G. Dongol, R. Wartchow, H. Butenschön, Eur. J. Org. Chem. 2002, 1972-1983.
7. D. Leinweber, R. Wartchow, H. Butenschön, Eur. J. Org. Chem. 1999, 167-179.