Maciej Cierpucha, Marek Chmielewski
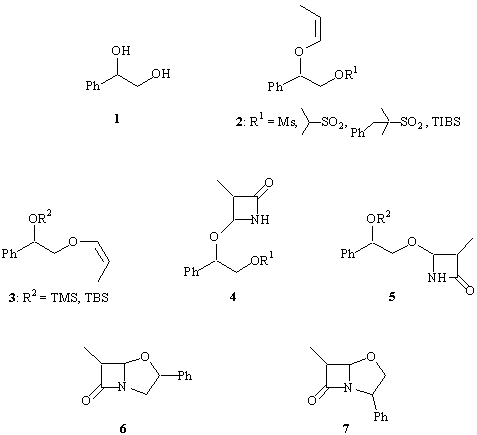
Both enantiomers of 2-phenyl-ethanediol 1 were transformed into corresponding (Z)-propenyl ethers 2 and 3. Diastereoselectivity of [2+2]cycloaddition of suitable protected 2 and 3 with chlorosulfonyl isocyanate was studied. β-Lactams 4 and 5, thus obtained, were subjected to the intramolecular alkylation of nitrogen atom to form title clavams 5 and 6. In both cases cycloaddition reactions are sterically controlled. Biological activities of isomeric clavams were investigated.
