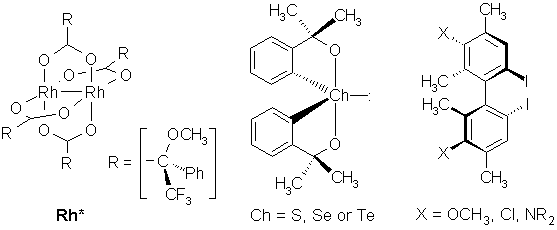
Recognition of axially chiral compounds with soft-base functionalities by the dirhodium method
Helmut Duddeck, a; S. Moeller a; A. Simon b; G. Tóth b; J. Drabowicz c
and K. Micha³ Pietrusiewiczd
a Institut für Organische Chemie, Universität Hannover, Germany
b Institute for General and Analytical Chemistry of the Budapest University of Technology and Economics, Budapest, Hungary
c Centre of Molecular and Macromolecular Studies, Polish Academy of Sciences, Department of Organic Sulfur Compounds, £ód, Poland
d Maria Curie-Sklodowska University, Department of Organic Chemistry, Lublin, Poland, and Polish Academy of Sciences, Institute of Organic Chemistry, Warsaw, Poland

Spirochalcogenuranes as shown in the formula scheme contain hypervalent chalcogen atoms with trigonal bipyramidal geometry and exhibit chirality even if both ligand arms are equal. They act as strong donors and form diastereomeric adducts with the chiral enantiopure dirhodium complex Rh*. The individual adduct species can be identified and stereochemically assigned by low-temperature 1H NMR spectroscopy. Recording 1H NMR spectra of these adducts is an excellent method for chiral discrimination in this class of compounds. In 2:1-ligands each ligand molecule attached to one rhodium atom can recognize which enantiomer is at the other rhodium on the back-side of the dirhodium complex cage. The signs of dispersion effects (non-racemic mixtures) indicate that establishing a rule for the determination of absolute configurations is possible in the spirochalcogenurane system. Some additional evidence about the structure of the adducts emerges from NOE experiments; the spatial contact of the geminal methyl groups in the spirochalcogenuranes depends strongly of their overall size.
Alkyl and aryl iodides are unpolar compounds which refuse to establish stable interactions with conventonal NMR auxiliaries. However, the soft acid dirhodium complex Rh* is capable of form labile adducts with organoiodine compounds although iodine is only a weak donor. Diastereomeric dispersions of room-temperature 1H NMR signals can be observed after mixing bis(2-iodophenyl) derivatives with an equimolar of Rh* ranging up to 10 Hz (at 400 MHz field strength).