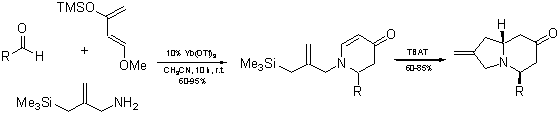
Asymmetric Synthesis of Multisubstituted Indolizidines by Intramolecular Addition of Allylsilane to Chiral 2,3-Dihydro-4-pirydones
Magdalena Dziedzic, Bartłomiej Furman
Institute of Organic Chemistry, Polish Academy of Sciences, Warsaw, Poland
Indolizidines are one of the most important classes of azabicyclic alkaloids, known for their wide range of physiological activities.1 Among methods of their synthesis, the intramolecular alkylation of enone moiety in dihydropyridones has been recently reported.2 Recently, we have described3 an efficient procedure for the stereoselective construction of multi-substituted indolizidines.
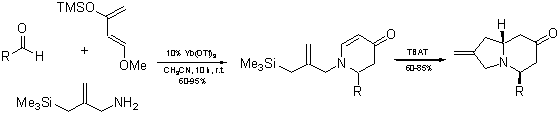
The discovered reaction sequence consists of the Lewis acid-mediated imino-Diels-Alder reaction followed by the intramolecular allylation of 2,3-dihydro-4-pyridones with allylsilane located in the side-chain. The final step of the overall process involves a newly developed method for the effective intramolecular conjugate addition of allylsilane to the dihydropyridones. We have found that in the presence of tetrabutylammonium triphenyldifluorosilicate (TBAT)4, the cyclocondensation proceeds in a good yield, while the results of Lewis acid-catalyzed allylation are generally not preparatively attractive.
These positive results encouraged us to initiate a project aimed at the enantioselective synthesis of indolizidines. In this communication, the results of our investigations will be presented.
1. (a) Aronstam, R. S.; Daly, J. W.; Spande, T. F.; Narayanan, T. K.; Albuquerque, E. X. Neurochem. Res. 1986, 11, 1227. (b) Daly, J. W.; Garraffo, H. M.; Spande, T. F. In The Alkaloids; Cordell, G. A., Ed.; Academic Press: San Diego, CA, 1993; Vol. 43, pp. 185-288
2. (a) Beckwith, A. L. J.; Joseph, S. P.; Mayadunne, T. A. J. Org. Chem. 1993, 58, 4198. (b) Comins, D. L.; Zhang, Y. J. Am. Chem. Soc. 1996, 118, 12248.
3. Furman, B.; Dziedzic, M. Tetrahedron Lett. 2003, 44, 6629.
4. Pilcher, A. S.; Ammon, H. L.; DeShong, P. J. Am. Chem. Soc. 1995, 117, 5166.