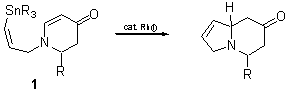
Rhodium-Catalyzed Intramolecular Conjugate Addition of Vinylstannates to 2,3-Dihydro-4-pyridones
Institute of Organic Chemistry, Polish Academy of Sciences, Warsaw, Poland
The 1,4-conjugate addition of organometallic reagents to α,β-unsaturated carbonyl compounds is an important process for carbon-carbon bond formation.1 Such reactions are typically carried out using organocopper reagents. Recently, Miyaura has reported the addition of organoboronic acids to α,β-unsaturated carbonyl compounds catalyzed by rhodium complexes.2 Furthermore, Inoue and Oi have reported the rhodium-catalyzed conjugate addition of organostannanes to α,β-unsaturated ketones and esters.3 In these catalytic reactions, organorhodium complexes generated by the transmetalation from the organometallic precursors are assumed to be the active species which react with the substrates.

These results prompted us to investigate whether the corresponding 2,3-dihydro-4-pyridones of the type 1 could be substrates in the rhodium-catalyzed intramolecular conjugate addition with side-chain located vinyltin reagents. Herein we wish to report, experimental details as well as scope and limitations of this novel cyclocondensation.
1. Rossiter, B. E.; Swingle, N. M. Chem. Rev. 1992, 92, 771.
2. (a) Sakai, M.; Hayashi, T.; Miyaura. N. Organometallics 1997, 16, 4229. (b) Takaya, Y.; Ogasawara, M.; Hayashi, T.; Sakai, M.; Miyaura. N. J. Am. Chem. Soc. 1998, 120, 5579.
3. Oi, S.; Moro, M.; Ono, S.; Inoue, Y. Chem. Lett. 1998, 83.