Synthesis and Structures of Some
Di- and Triferrocenylmethane Derivatives
Josep Ramon Garabatos Perera, Holger Butenschön
Institut für Organische Chemie, Universität Hannover, Germany
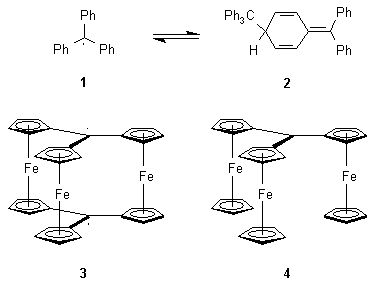
The triphenylmethyl system has attracted the interest of organic chemists since the days of Gomberg, who first postulated the existence of the triphenylmethyl radical (1) and the reversible equilibrium with its dimer.1 However, it was not until 68 years later, that it was found coclusively, that it does not dimerize with formation of hexaphenylethane but to give the quinoid dimer 2 after recombination.2
The free carbon radicals are highly reactive species. Nevertheless, some of them show a remarkable thermal and chemical stability governed by two main factors: resonance and steric hinderance. Since Bunz reported evidence of ferrocene being more aromatic than benzene,3 a comparison of the triferrocenylmethyl system 3 and 4 with 1 is of interest with respect to the chemistry at the quasi-benzylic carbon atom.

In the context of multiply bridged ferrocenes, with the ultimate goal of a synthesis of 3 and derivatives, some triferrocenylmethane derivatives were prepared by reaction of triferrocenylmethanol with triphenylcarbenium tetrafluoroborate followed by a nucleophile. Treatment of 1,1’-bis(tributylstannyl)ferrocene with 1 equiv. of butyllithium followed by chloroethylformiate affords complexes with one (1-ethoxycarbonyl-1’-tributylstannyl-ferrocene), two (di[1,1’-(tributylstannyl)ferrocenyl]methanone) or three ferrocenyl units (tris[1’-(tributylstannyl)ferrocenyl]methanol). The last is the first triferrocenylmethane derivative with substituents at the opposite cyclopentadienyl ring. Threefold lithiation of this compound is shown to work using butyllithium followed by dimethylformamide.4
1. M. Gomberg, J. Am. Chem. Soc. 1900, 22, 757;
3. M. Laskoski, W. Steffen, M. D. Smith, U. H. F. Bunz, Chem. Commun. 2001, 691-692.
4. J. R. Garabatos, H. Butenschön, submitted.