TRIANGLIMINES AND TRIANGLAMINES
Jacek Gawroński
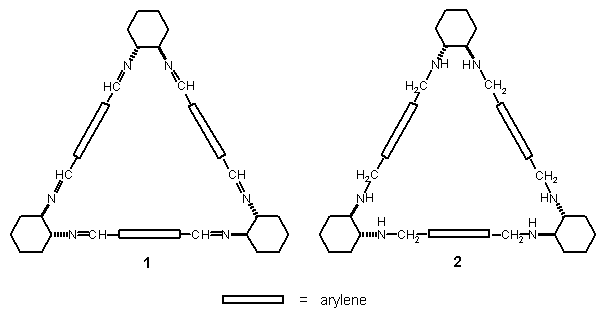
Trianglimines (1) are chiral cyclic hexaimines obtained from 3+3 cyclocondensation of aromatic dialdehydes and trans-1,2-diaminocyclohexane.1 These compounds result from conformationally and thermodynamically driven reaction pathway and are in many cases the only or the dominating products, with little or no polymeric byproducts formed. Many dialdehyde substrates of differing structures have been used, including para and meta isomers.2 It appears that in the case of less rigid dialdehyde substrate the products may include also 2+2 and other cyclocondensation components.
The focus of the presentation will be on the following issues:
● the effect of dialdehyde structure on the oligoimine products distribution
● the structure of the trianglimines and other cyclic oligoimines
● the applications of trianglamine 2, the product of reduction of 1, and its derivatives.

1. J. Gawroński, H. Ko³bon, M. Kwit, A. Katrusiak, J. Org. Chem. 2000, 65, 5768.
M. Chadim, M. Budĕinskij, J. Hodačovį, J. Zįvada, P. C. Junk, Tetrahedron: Asymmetry, 2001, 12, 127.
2. N. Kuhnert, Ch. Straβnig, A. M. Lopez-Periago, Tetrahedron: Asymmetry 2002, 13, 123.
N. Kuhnert, A. M. Lopez-Periago, Tetrahedron Letters, 2002, 43, 3329.
N. Kuhnert, G. M. Rossignolo, A. M. Lopez-Periago, Org. Biomol. Chem. 2003, 1, 1157.
M. Kwit, J. Gawroński, Tetrahedron: Asymmetry 2003, 14, 1303.
J. Gao. A. E. Martell, Org. Biomol. Chem. 2003, 1, 2795.