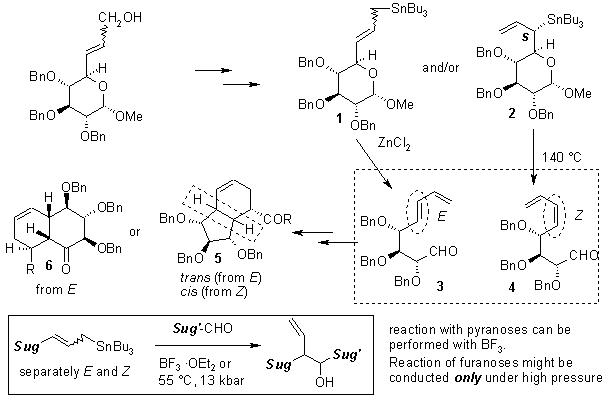
Sugar allylic alcohols are easily converted into the primary and/or secondary allyltin derivatives 1 and/or 2.1 Zinc chloride induces a rearrangement of both regioisomers (2, 3) into the E-dieno-aldehyde 3 exclusively,1,2 while secondary organometallics 2 decompose at high temperature into the Z-product 4.2 Primary analogue 1 is stable up to at least 170 °C.2

These dienoaldehydes were used for stereoselective preparation of complex, highly oxygenated carbobicyclic derivatives 4 and 5.3
Alternatively, primary sugar allyltins (derived from furanoses and pyranoses) were used for the preparation of homoallylic alcohols by reaction with sugar aldehydes.4
1. S. Jarosz, Tetrahedron, 1997, 53, 10765 and references therein.
2. S. Jarosz, K. Szewczyk, A. Zawisza, Tetrahedron: Asymmetry, 2003, 14, 1715.
3. S. Jarosz, E. Kozłowska, A. Jeżewski, Tetrahedron, 1997, 53, 10775; S. Jarosz, S. Skóra, Tetrahedron: Asymmetry, 2000, 11, 1425; S. Jarosz, K. Szewczyk, A. Zawisza, Tetrahedron: Asymmetry, 2003, 14, 1709.
4. S. Jarosz, S. Skóra, K. Szewczyk, Z. Ciunik, Tetrahedron: Asymmetry, 2001, 12, 1895; S. Jarosz, K. Szewczyk, R. Luboradzki, A. Gaweł, Tetrahedron: Asymmetry, 2004, 15, in press