Cyclooctenones via Claisen Rearrangement of Carbohydrate Derivatives
Stefan Jürs, Joachim Thiem
Highly complex substituted and in particular polyoxygenated eight-membered carbocycles occur in numerous natural products. This contribution reports on the use of carbohydrates as starting materials for the construction of such components. In addition to their use in natural product synthesis these compounds are also worth to be investigated with regards to non-hydrolysable carbohydrate mimetics.
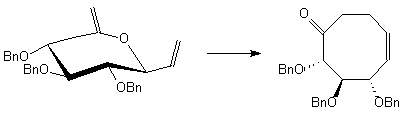
In a linear approach glucose was transformed into a cyclic allylvinyl ether precursor that could be converted via [3,3]-sigmatropic rearrangement into the corresponding cyclooctenone derivative. The most notable structural feature of this compound is the boat-chair conformation that could be elucidated by NOE spectroscopy. Subsequent functionalisations such as reduction, epoxidation, dihydroxylation and glycosylation proceeded highly diastereoselective.
