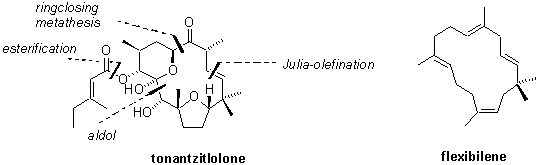
In 1997 Jakupovics and Jeske isolated a new diterpenoid from the endemic Mexican plant Stillingia sanguinolenta and named it tonantzitlolon 1.1 Besides flexibilene 2 it is the only 15-membered macrocyclic diterpene found in nature so far. Preliminary biological evaluation at the NCI indicated high activity and selectivity against human breast and kidney cancer cell lines. The novel highly complex and unique structure together with its promising biological activity makes 1 an ideal candidate for total synthesis.
The report will focus on the modular and convergent total synthesis of this novel diterpenoid. Preliminary biological data will be disclosed which shed light on the possible cellular target of tonantzitlolone.
F. Jeske, Ph. D. thesis, TU Berlin 1997.