Asymmetric Syntheses of Substituted N-Heterocycles Using
Glycosylamines as Chiral Auxiliaries
Stephan Knauer, Horst Kunz
Institut für Organische Chemie, Johannes Gutenberg-Universität Mainz, Germany
In search of novel lead structures for drug development, one consistently comes across the piperidine ring system. Therefore, the stereoselective synthesis of these heterocyclic structures with varying substitution patterns is of general interest in medicinal chemistry.
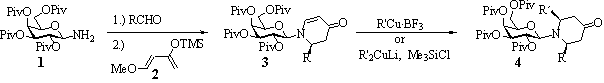
During the past years, we developed a strategy for the enantioselective synthesis of piperidine alkaloids using glycosylamines as chiral auxiliaries.1,2 The condensation of 2,3,4,6-tetra-O-pivaloylated d-galactosylamine 1 with a corresponding aldehyde and subsequent Lewis acid-catalysed reaction with 1-methoxy 3-trimethylsiloxy butadiene 2 delivered the 2-substituted 5,6-dehydropiperidinone derivatives 3 in excellent yield and with high stereoselectivity.

The obtained dehydropiperidinones 3 offer numerous possibilities to introduce additional substituents, such as conjugate 1,4-addition of organocuprates. Due to the reduced reactivity of the enaminone structure, the addition required Lewis acid-activation or pre-treatment with chlorotrimethylsilane to yield the 2,6-cis-disubstituted piperidinones 4 in high diastereoselectivity.2,3 Inverse configuration in 2- and 6-position can be achieved using N‑arabinosylamine instead of the galactosyl auxiliary.4
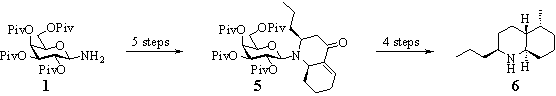
Starting from the protected galactosylamine 1 we were able to generate both trans- and cis-annulated decahydro quinoline derivates.5 The synthesis of trans-4a-epi-pumiliotoxin C 6, an alkaloid of the poison dart frogs of the family Dendrobates, illustrates this concept of asymmetric induction using carbohydrate auxiliaries.
1. H. Kunz, W. Pfrengle, Angew. Chem. 1989, 101, 1041; Angew. Chem Int. Ed. 1989, 28, 1067.
2. M. Weymann, W. Pfrengle, D. Schollmeyer, H. Kunz, Synthesis 1997, 1151.
3. H. Kunz, M. Weymann, M. Follmann, P. Allef, K. Oertel, M. Schultz-Kukula, A. Hofmeister, Polish J. Chem. 1999, 73, 15.
4. H. Kunz, M. Weymann, M. Follmann, P. Allef, K. Oertel, M. Schultz-Kukula, A. Hofmeister, Polish J. Chem. 1999, 73, 15.
5. a) M. Weymann, M. Schultz-Kukula, H. Kunz 1998, 39, 7835; b) M. Weymann, M. Schultz-Kukula, S. Knauer, H. Kunz, Monatshefte Chemie 2002, 133, 571.