Wioletta Kośnika, Andrew Stachulskyb, M. Chmielewskia
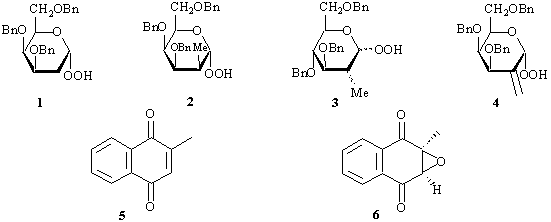
Anomeric hydroperoxides 1-4 derived from 2-deoxy-hexoses were found to be stable enough to be purified by chromatography. Enantiomeric epoxidation of 2-methyl-1,4-naphtoquinone (5) with 1 in the presence of DBU provided corresponding epoxide 6 with ee = 43% whereas the same reaction using 2 provided 6 with ee = 47% and using 4 with ee=22% only.
The aim of the study is to find sugar that can be oxidized to single anomeric hydroperoxide, α or β, which after epoxidation of the quinone could be isolated and reoxidized.
