Enantioselective allylation of glyoxylates catalysed by (salen)chromium(III) complexes
Piotr Kwiatkowski,a Wojciech Chaładaj,b Janusz Jurczaka,b
The addition of allylic reagents to aldehydes or ketones leads to very important, from synthetic point of view, homoallylic alcohols. Many enantioselective catalytic systems have been developed until now especially for allylation of simple aromatic and aliphatic aldehydes.1
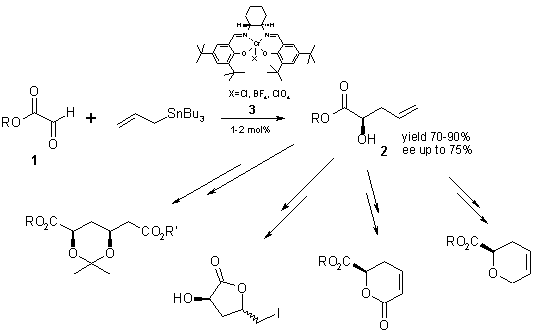
We focused our attention on enantioselective allylation of activated aldehydes, such as alkyl glyoxylates 1 leading to compounds of type 2, interesting precursors for synthesis of various natural products.

The addition of allylstannanes and allylsilanes to glyoxylates of type 1, catalyzed by various chiral metalosalen complexes, has been studied. We have found that the reaction proceeds smoothly with allyltributyltin, and low loading of (salen)Cr(III)X 3 (X= BF4, ClO4) affording 2‑hydroxypent-4-enoic acid esters 2 with good yield (70-90%) and enantioselectivity 58-75% ee. An influence of experimental variables, such as solvents, temperature, quantity of the catalyst 3, structure of the salen ligand, and concentration of glyoxylate 1, has been also studied.
1. For a recent review, see: S. E. Denmark, J. Fu Chem. Rev. 2003, 103, 2763-2793.