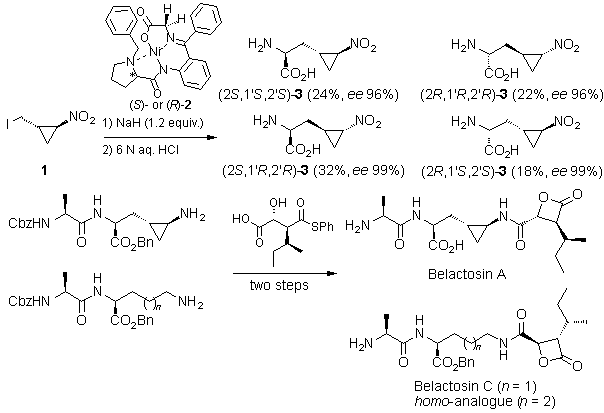
Asymmetric Synthesis of All Four Diastereomers of 3-(trans-2-Nitrocyclopropyl)alanine and First Enantioselective Total Syntheses of Belactosin A, Belactosin C, and its Homo-Analogue
All four diastereomers of 3-(trans-2-nitrocyclopropyl)alanine 3 were prepared by asymmetrically induced a-C alkylation of the glycine moiety in the (S)- or (R)-Ni(II)-Schiff base complex (S)-2 or (R)-2, employing racemic trans-1-iodomethyl-2-nitrocyclopropane (1) as the alkylating agent.1

Catalytic hydrogenation of (2S,1'R,2'S)-3 led to 3-(trans-2'-aminocyclopropyl)alanine,2 a constituent of the natural product Belactosin A, a highly potent proteasome inhibitor. The final assembly of Belactosin A as well as two other members of the belactosin family (Belactosin C and its homo-analogue) has been accomplished in an enantioselective manner with high overall yields. The developed concise approach comprises a novel sequential acylation / b-lactonization reaction, and allows a facile alteration of the substituents, thus providing a flexible route to the new family of highly active belactosin-based proteasome inhibitors.3
1. O. V. Larionov, T. F. Savel'eva, K. A. Kochetkov, N. S. Ikonnokov, S. I. Kozhushkov, D. S. Yufit, J. A. K. Howard, V. N. Khrustalev, Y. N. Belokon, A. de Meijere, Eur. J. Org. Chem. 2003, 869–877.
2. O. V. Larionov, S. I. Kozhushkov, M. Brandl, A. de Meijere, Mendeleev Commun. 2003, 199–200.
3. O. V. Larionov, A. de Meijere, Org. Lett. 2004, submitted.