LIGATION
OF
PEPTIDES
via
RING
OPENING
CROSS
METATHESIS
(ROCM)
Simon Michaelis,
Siegfried Blechert
Institut für
Organische Chemie, Technische Universität Berlin, Germany
Introduction
Regarding the development of
new medical therapeutics, have recently peptide mimetics increased in
importance. To synthesize mimetics, it is important to have appropriate
chemical methods for the cell-free synthesis of peptides to expand the
repertoire of ribosomal synthesis to include non-coded amino acids and other
structural compounds. To prepare biologically relevant peptide domains,
consisting of 130 (±40) amino acids, one must first synthesize building
blocks of about 50 amino acids by solution or by solid-phase methods
according to Merrifield. These peptide units can be stitched afterwards by
using several different ligation techniques, which require the presence of
certain functional groups (here X and Y).[1]
Amongst other kinds of
metathesis reaction, the ring opening cross metathesis (ROCM)[2] is
offering an interesting possibility to connect C-C bonds. Cyclic systems
which are under tension can be converted by ring opening metathesis and
following cross metathesis with olefinic cross partners into ring-opened
products. The driving force of this atom economic metathesis reaction is the
decrease of the ring-tension. ROCM is characterized by its high efficiency
providing high yields and the use of smallest amounts of metathesis
catalysts.
Concept
The following concept of
ligating the peptide units by ROCM offers the big advantage, that apart from
the double bound no other functional groups are needed, which is unique
amongst the other known techniques of ligation.
Cyclus
2 is
characterized by a high ring tension which should allow ROCM with the
metathesis partner 1 with smallest amounts of a catalyst.
Furthermore, after the coupling of the two groups R and R´ a structure
similar to proline is introduced at the site of ligation.
If the two groups R and
R` are peptide units a ligation of these two segments is achieved by ROCM.
Because of the high symmetry of the cyclus only one regioisomer is
guaranteed.
It is also known, that the
specific introduction of additional structure units at the site of ligation
has effects on the tertiary structure of the peptide[3]. As
proline is found abundantly in regions of ß-turns, the introduction of a
proline-like structure could initiate the formation of a ß-turn.
Synthesis of the precursors for ROCM
The cyclic amine 5 can be synthesized by endoselective Diels-Alder reaction[4] from
cyclopentadiene 7 and maleimide 8 to the imide 6
and reduction with lithium aluminium hydride.[5] The
coupling of the cyclus 5 with a peptide segment can be realized by
reacting the amine function of 5 and the acidic function of the
peptide segment with coupling reagents (e.g. DCCI / DMAP or TBTU).[6]
Beneath the N- and the
C-terminus of the peptide the side chains of amino acids are also available
to introduce the double bond in order to guarantee a peptidic partner for
metathesis. A simple method using the C-terminus, is esterification of an
olefinic alcohol with the acidic function of a peptide, while allylglycine
can introduce the double bond via its side chain.
Ring opening cross metathesis
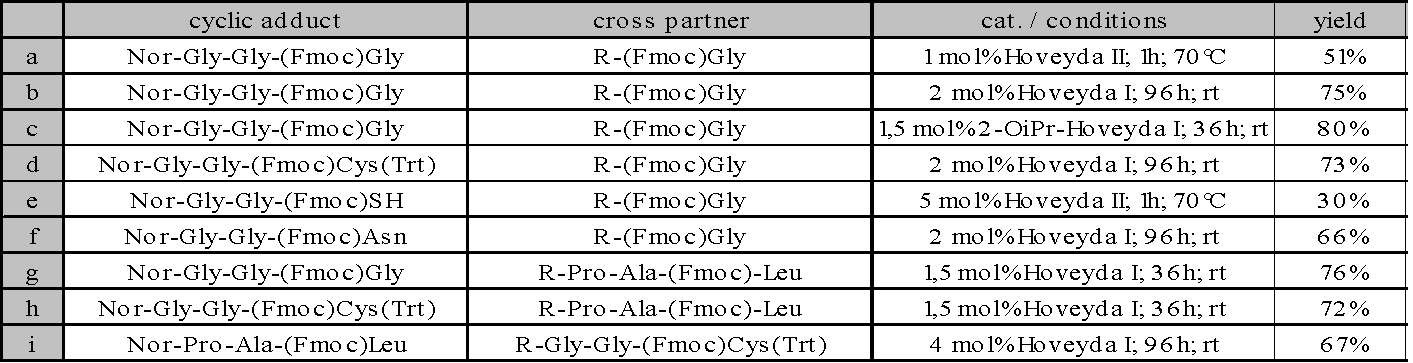
The ROCM of Nor-Gly-Gly-(Fmoc)Cys(Trt)
10 with the coupling partner
R-Pro-Ala-(Fmoc)Leu 9 shown above could be done by using 1,5 mol% of
the Hoveyda catalyst of the first generation within 36h at room temperature,
giving a good yield of 72% as an example.
The following table is to
demonstrate the tolerance of the ruthenium catalyst against the functional
groups of certain amino acids. It could be seen, that only strongly
nucleophilic functional groups such as NH- or SH-groups interfered with
metathesis. Yet even reactions with free SH-groups were successfully run if
the Hoveyda II-calalyst was used.
Generally Hoveyda I showed to
be superior to Hoveyda II and the Grubbs catalysts, as the more active
catalysts lead to polymeric by-products. A modification of the Hoveyda
I-catalyst, leading to the more active 2-OiPr-Hoveyda I-catalyst[7], developed
within the group of S. Blechert could even outperform the established
catalysts in the regarded case.
Currently we are examining the
influence of peptide segments to the stereochemistry of the ring-opening
during the ROCM. We are also working towards asymmetric ROCM with chiral
metathesis catalysts[8] as
well as ROCM in aqueous mediums.
1 P. E. Dawson, S.
H. Kent, Annu. Rev. Biochem. 2000, 69, 923.
2a)
A. Fürstner, Angew. Chem. Int. Ed. Engl. 2000, 39,
3013; b) M. Schuster, S. Blechert, Angew. Chem. Int. Ed. Engl.
1997, 36, 2036. c) M. F.
Schneider, S. Blechert, Angew. Chem. Int. Ed. Engl. 1996,
55, 410.
3[3
U. Nagai, K. Sato, Tetrahedron Lett.
1985, 26,
647.
4
S. Harvy, J. Amer. Chem. Soc.
1949, 71,
1121
5 Y. L. Chow, C. L.
Colon, Acta Chem. Scandinavia 1982, 36, 623
6 M. Bodanszky, A.
Bodanszky, The Practice of Peptide Synthesis 2nd, Springer Lab
Manual, 1994
7
M. Zaja, S. J. Connon, A.
M. Dunne, M. Rivard, N.
Buschmann, J. Jiricek,
S. Blechert, Tetrahedron 2003, 59, 6545
8
J. J. van Veldhuizen, D. G. Gilligham, S. B. Garber, O. Kataoka,
A. H. Hoveyda, J. Amer. Chem. Soc.
2003, 125,
12502

