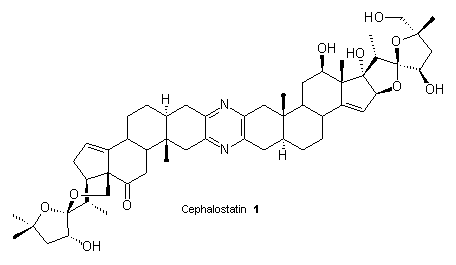
Approaches towards the synthesis of new natural products with cytostatic activity
Secondary metabolites of plants and animals continue to attract attention of organic chemists, biochemists, and pharmacologists due to their interesting structures and potent biological activities. One such example is cephalostatin 1 isolated from the Indian Ocean hemichordate Cephalodiscus gilchristi, which exhibited remarkable cytotoxic activity against a broad spectrum of malignant tumor cells. Similar marine alkaloids (e.g. ritterazine G) were found in

the lipophilic extract of the tunicate Ritterella tokioka collected off the coast of Japan. These very potent compounds, cephalostatins and ritterazines, belong to the large family of trisdecacyclic pyrazines consisting of two steroid units. The biological activity of the dimeric steroid-pyrazine marine alkaloids and their limited availability coupled with the new and intriguing structure make them an attractive challenge for the synthetic organic chemists. New methods of synthesis of the pyrazine dimers will be presented.
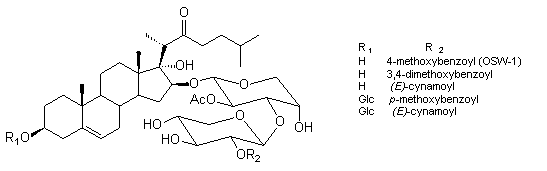
A few years ago a group of cholestane glycosides was isolated from the bulbs of Ornithogalum saundersiae, a species of the lily family without any medicinal folkloric background. Similar glycosides were recently isolated from Galtonia candicans. The major component of the mixture of saponins, OSW-1, exhibited sub-nanomolar antineoplastic activity. While OSW‑1 is exceptionally cytotoxic against various tumor cells, it showed little toxicity to normal human pulmonary cells. The cytotoxicity profile of OSW-1 against different cancer cell lines was found to be surprisingly similar to that of the cephalostatins, which appears to imply a related mechanism of action. The methods of synthesis of saponin OSW-1 and its analogues, as well as the structure-activity relationship will be discussed.