 1
1
 2
2
Institut für Organische Chemie, Universität Hannover, Germany.
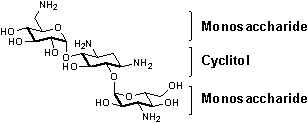
Changing the conformation of nucleic acids at will would enable man to interfere with the life cycle of cells and viruses. RNA with its huge conformational variety (e.g. TAR RNA of HIV‑1) is a very promising target for such an approach. Aminoglycosides like Kanamycin 1 are prominent for their good binding properties to RNA.
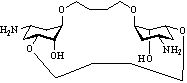
This project focuses on novel aminocyclitols and their chemical and biological behavior, while aminosugars are already part of studies in our group1-3. In this context, our efforts are governed by the goal to design novel „artificial“ macrocycles like 2. These novel structures consist of rigid cyclohexane rings which are connected to each other by a flexible linker.
 1
1
 2
2
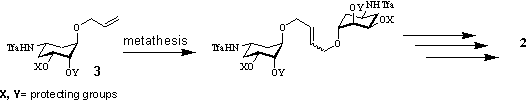
Their oligomeric character containing several amino groups is essential for efficient binding and should lead to cooperative effects and hence tighter binding. Their synthesis is achieved by metathesis reactions starting from allyl linker building blocks like 3. This synthesis strategy yields extended carbaaminosugar structures like 2 in a few steps.
1. A. Kirschning, G.-w. Chen, Tetrahedron Lett. 1999, 40, 4665-4668.
2. A. Kirschning, G.-w. Chen, Chem. Eur. J. 2002, 8, 2717-2729.
3. A. Kirschning, M. Lindner, Tetrahedron 2004, 60, 3505-3521.