Carbohydrates as Polyfunctional Scaffolds for Combinatorial Chemistry
Till Opatz, Horst Kunz
Institut für Organische Chemie, Johannes Gutenberg-Universität Mainz, Germany
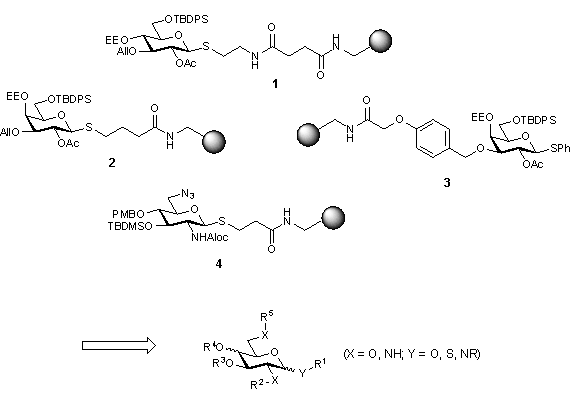
Their high density of functional groups and their availability in a number of diastereomeric forms should make monosaccharides ideal scaffolds for combinatorial chemistry, which allow the attachment of up to five different substituents. However, a carefully chosen set of selectively removable protecting groups in combination with a cleavable anchor is required if selective manipulations are to be carried out on solid support.

Glycosides 1 to 4 have been used successfully as polyvalent scaffolds for the parallel solid phase-synthesis of compound libraries. Depending on the substituents attached to the hydroxy functions, the products can serve as, for instance, peptidomimetics, artificial receptors or RNA-ligands.1-6
1. C. Kallus, T. Opatz, T. Wunberg, W. Schmidt, S. Henke, H. Kunz, Tetrahedron Lett. 1999, 40, 7783-7786.
2. T. Opatz, C. Kallus, T. Wunberg, W. Schmidt, S. Henke, H. Kunz, Carbohydr. Res. 2002, 337, 2089-2110.
3. T. Opatz, C. Kallus, T. Wunberg, W. Schmidt, S. Henke, H. Kunz, Eur. J. Org. Chem. 2003, 1527-1536.
4. T. Opatz, C. Kallus, T. Wunberg, H. Kunz, Tetrahedron 2004 (Symposium in print), in press.
5. U. Hünger, J. Ohnsmann, H. Kunz, Angew. Chem. Int. Ed. Engl. 2004, 43, 1104-1107.
6. T. Maidhof, Dissertation, Universität Mainz, 2002.