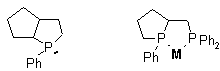
Bicyclo[3.3.0]octane Structural Motif in P-Stereogenic Ligand Design
K. Michał Pietrusiewicz,a,b Piotr Osiński,a Zbigniew Pakulski,a Oleg Demchuka
aInstitute of Organic Chemistry, Polish Academy of Sciences, Warsaw, Poland
b Department of Organic Chemistry, Maria Curie-Skłodowska University, Lublin, Poland
Asymmetric synthesis mediated by transition metals bearing chiral phosphine ligands has become a cornerstone of organic chemistry. Much effort has been directed towards the design, synthesis, and testing of new chiral phosphines for various synthetic purposes. For purely preparative reasons, the vast majority of the available chiral phosphine and diphosphine ligands are predicated upon carbon based central or axial chirality rather than asymmetry about the tetrahedral phosphorus. However, recent advances in the stereoselective synthesis and resolutions of P-stereogenic phosphorus compounds have opened the way also to a rational design of ligands bearing P-centered chirality and the number of highly successful P-stereogenic ligands on record is continuously growing.1 Especially successful have proven those possessing a five-membered ring as the key structural motif.2,3
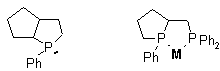
We shall report that novel monodentate and bidentate ligands
incorporating a P-stereogenic center embeded in the five-membered ring of the
phosphabicyclo[3.3.0]octane ring or chelate system can be readily synthesized
from simple phospholene precursors. The use of synthesized ligands as highly
efficient chiral auxiliaries in allylic substitution reactions and in transition
metal-catalyzed hydrogenations affording up to 98% ee will be demonstrated.
The scope and the stereochemical aspects of the synthesis of the enantiopure ligands and dependence of their catalytic performance on the configuration of the ring junction in the phosphabicyclo[3.3.0]octane skeleton will be discussed in details. The modified ‘quadrant rule’4 allows to explain the sense of the observed asymmetric induction.
1. Crépy K. V. L., Imamoto T. Top. Cur. Chem. 2003, 229, 1.
2. Tang W., Zhang X. Angew.Chem. Int. Ed. Engl. 2002, 41, 1612.
3. Shimizu H., Saito T., Kumobayashi, H. Adv. Synth. Catal. 2003, 345, 185.
4. Gridnev I. D., Higashi N., Asakura K., Imamoto T. J. Am. Chem. Soc. 2000, 122, 7183. Tang W., Zhang X. Chem. Rev. 2003, 103, 3029.