Synthesis of Heterocycles and Natural Products via Metalated Alkoxyallenes
Hans-Ulrich Reissig
Institut für Chemie, Freie Universität Berlin, Germany
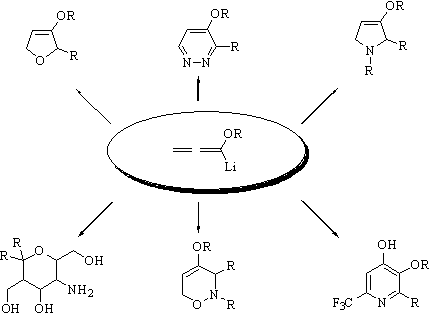
Lithiated alkoxyallenes are easily generated by treatment of the corresponding allenes with n‑butyllithium and they subsequently react with a variety of electrophiles giving products with high synthetic versatility. Based on the results of Brandsma and Arens we developed stereoselective syntheses of enantiopure furanone derivatives. By oxidative cleavage and reaction with hydrazine dihydrofurans are converted into pyridazine derivates.
Reactions of lithiated alkoxyallenes with (chiral) imines allow a highly flexible approach to dihydropyrrole derivatives. The stereoselective preparation of the polyhydroxylated g-amino acid (-)-detoxinine will be presented as example for the application in natural product synthesis. Addition of lithiated methoxyallene to nitriles led to a serendipitous discovery of unusually substituted pyridines.

Use of nitrones as electrophiles allows a novel [3 + 3] cyclization stereoselectively leading to functionalized 2H-1,2-oxazines which serve as precursor for enantiopure amino alcohols, pyrrolidines, and pyran derivatives.
1. H.-U. Reissig, S. Hormuth, W. Schade, M. Okala Amombo, T. Watanabe, R. Pulz, A. Hausherr, R. Zimmer, Lectures in Heterocyclic Chemistry Vol XVI in J. Heterocycl. Chem. 2000, 37, 597 – 606; H.-U. Reissig, W. Schade, M. G. Okala Amombo, R. Pulz, A. Hausherr, Pure Appl. Chem. 2002, 74, 175 – 180.
2. R. Pulz, A. Al-Harrasi, H.-U. Reissig, Org. Lett. 2002, 4, 2353 – 2355; O. Flögel, M. G. Okala Amombo, H.-U. Reissig, G. Zahn, I. Brüdgam, H. Hartl, Chem. Eur. J. 2003, 9, 1405 – 1415.