Chiroptical Properties of Bi- and Tricyclic b-Lactam Derivatives. Structure – CD data Relationship
Patrycja Szczukiewicz, Jadwiga Frelek
Institute of Organic Chemistry, Polish Academy of Sciences, Warsaw, Poland
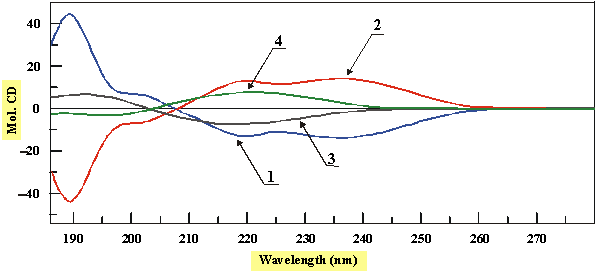
Determination of the absolute configuration at the bridge-head carbon atom in the b-lactam derivatives remains very important because of the close relationship between their structure and biological activity. Moreover, pyramidal hybridization of the b-lactam nitrogen has been found to be one of the factors that may increase the biological activity of b-lactam antibiotics. Recently, a helicity rule was established which allows stereochemical assignment to clavams and oxacephams on the basis of the CD data. The rule correlates a positive (negative) torsional angle of the amide unit with negative (positive) sign of the n®p* Cotton effect (CE).1,2

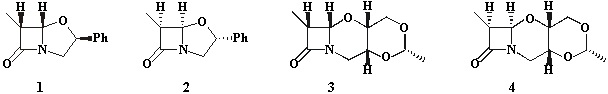
The aim of this work is to prove validity of the helicity rule for differently substituted clavams and oxacephams, some representative examples of which are presented above.
It has been found that investigated compounds follow the proposed helicity rule. Thus, compounds 2 and 4 with an (R) configuration at the ring junction display a positive CE associated with the n®p* amide transition whereas compounds 1 and 3 exhibit a negative CE for the same transition, in accordance with their (S) absolute configuration at the same carbon atom (see Figure below).