Synthesis and structure of axially chiral resorcin[4]arenes
Institute of Organic Chemistry, Polish Academy of Sciences, Warsaw, Poland
Helically and axially chiral molecules and assemblies are widely spread in biological systems. Some of them, like transmembrane channel proteins, combine the this feature with complexation ability.1 In contrary, synthetic systems that mimic the combination of those properties are rather scarce (examples involve C2-symmetrical crown ether–binapthol compounds2 or inorganic assemblies3). Thus, we aimed at constructing axially chiral compounds having inherent complexation properties.
Since amide-derivatized resorcin[4]arenes are known to show interesting complexation properties, taking advantage of both cation-pi and hydrogen bonding interactions,4,5 we decided to use that platform for the construction of chiral analogues. In the current presentation, results concerning diastereoselective synthesis of C4-symmetrical resorcin[4]arenes will be presented. The synthesis was preformed via direct Mannich reaction between resorcin[4]arene, formaldehyde and amino acid derivatives (Scheme). X-ray structure and CD spectra were used for the determination of the product’s structure (configuration and cavity size).
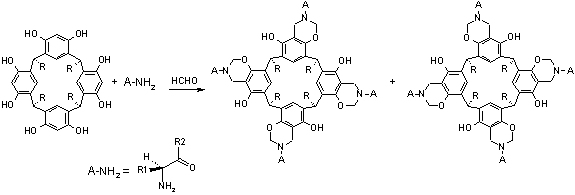
Scheme
1. D. A. Doyle, J. M. Cabral, R. A. Pfuetzner, A. Kuo, J. M. Gulbis, S. L. Cohen, B. T. Chait, R. MacKinnon, Science, 1998, 280, 69.
2. L. Pu, Chem Rev., 1998, 98, 2405.
3. K. Biradha, C. Seward, M. J. Zaworotko, Angew. Chem. Int. Ed., 1999, 38, 492.
4. J. L. Atwood, A. Szumna, J. Am. Chem. Soc. 2002, 124, 10646.
5. J. L. Atwood, A. Szumna, Chem. Commun. 2003, 940.