Convergent Syntheses of Some Essential Building Blocks for the Total Synthesis of Hormaomycin
Institut für Organische und
Biomolekulare Chemie, Georg-August-Universität Göttingen,
Germany
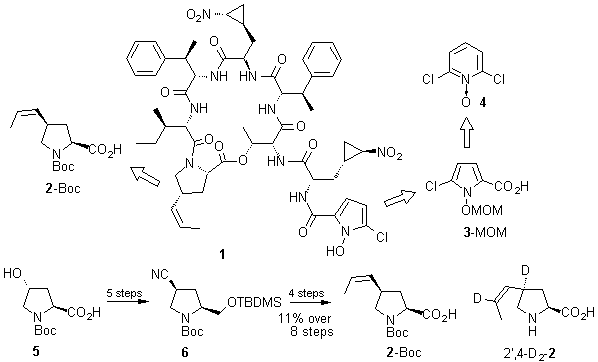
As a prerequisite for the total synthesis of hormaomycin 1 with its interesting spectrum of biological activities, and some of its analogs, productive syntheses of suitably protected 3-(2'-nitrocyclopropyl)alanine, as well as 5-chloro-1-hydroxypyrrol-2-carboxylic acid (3) and (2S,4R)-4-(Z)-propenylproline (2) have been developed. The O-MOM protected acid 3 was synthesized in 7 steps from 2,6-dichloropyridine-N-oxide 4 in 8% overall yield.

The N-Boc protected (2S,4R)-4-(Z)-propenylproline 2-Boc was obtained from the N-Boc-protected (2S,4R)-hydroxyproline 5 via the advanced intermediate 6, which is easily accessible on a 30-50 g scale, in 11% yield over 8 steps. The same intermediate was used as a starting material for the preparation of the doubly deuterium-labeled amino acid 2',4-D2-2 which was used for the elucidation of the absolute configuration of the propenylproline residue in hormaomycin 1.1 The final assembly of all the necessary amino acids and deprotection gave synthetic hormaomycin 1 which showed the same biological activity as the natural material obtained by fermentation.2
1. B. D. Zlatopolskiy, K. Loscha, H.-P. Kroll, M. Klingebiel, P. Alvermann, S. I. Kozhushkov, S. V. Nikolaev, A. Zeeck, A. de Meijere, Chem. Eur. J. 2004, submitted.
2. B. D. Zlatopolski, A. de Meijere, Chem. Eur. J. 2004, submitted.