The most important process
involving
benzosultams is an extrusion of sulfur dioxide leading to aza-ortho-xylylenes.
We investigated mass spectra of benzosultams and have found that,
usually,
a loss of SO2 from molecular ion resulting in the formation
of [M-64] ion is a predominant process.
We proposed
two mechanisms of elimination of SO2 from molecular ions of
benzosultams:
a) concerted mechanism with
simultaneous
break of sulfur-nitrogen and carbon-sulfur bonds resulting in direct
formation
of azaxylylene, and
b) stepwise mechanism in which the
carbon-sulfur bond is broken first, and [1,5] hydrogen shift precedes
elimination
of SO2

The molecular ion of
cyclopropanespiro-benzo-sultam
shows much more complex fragmentation pattern, formation of [M-64] ion
proceeds to a minor extent.
In
4-nitro-3-cyclopropanespirobenzosultam
the initial fragmentation involves interaction of methylene and nitro
groups
and results in elimination of formaldehyde
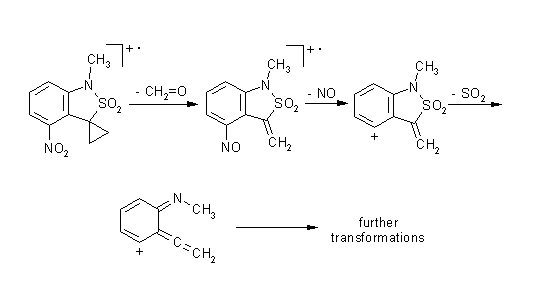
. In cyclobutanespirobenzosultam the [2+2] cycloreversion resulting in elimination of ethylene precedes the elimination of SO2.
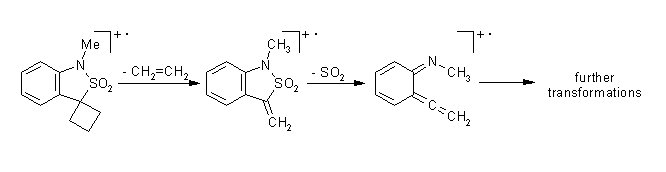
W. Danikiewicz, K. Wojciechowski, S. Kosiński, and M. Olejnik J. Mass Spectrom. 2001, 36, 430.
In N-(methoxymethyl)-benzosultams a novel rearrangement resulting in an elimination of formaldehyde was observed. For this process we proposed a mechanism involving a presence of an ion-neutral complex.
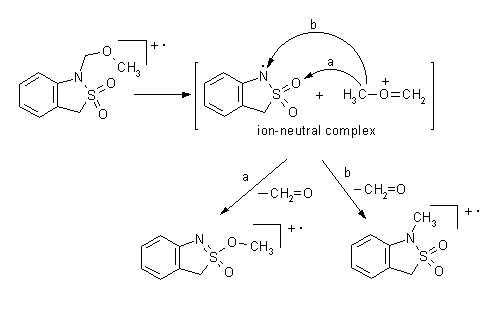
W. Danikiewicz and K. Wojciechowski Rapid. Commun. Mass Spectrom. 1996, 10, 36.
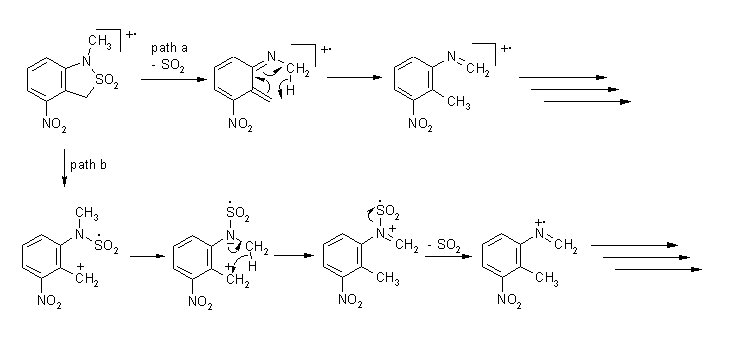
In 1-alkyl-7-nitrobenzosultams primary fragmentations involve interaction of N-alkyl and nitro group resulting in an elimination of carbonyl compounds.
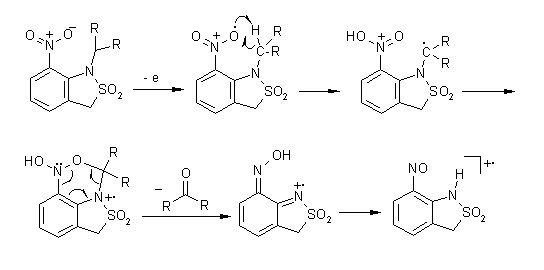
W. Danikiewicz and K. Wojciechowski Eur. Mass Spectrom. 1997,
3,
55.