Grant OPUS 2020/37/B/ST4/00017 funded by National Science Centre.
Realizaton period: 2020-2024
For many applications of organic semiconductors, planarity is considered a prerequisite. This is largely because key properties (such as a low HOMO–LUMO gap and polarizability) are dependent on strong π-conjugation. Deviation from planarity, however, can give rise to new and exciting electronic, optical, and magnetic properties that are currently unknown or underinvestigated in the field of organic semiconductors, but possess considerable potential. In addition, non-planarity can produce helically-chiral structures, with chiroptical properties. Helical polycyclic aromatic hydrocarbons (PAHs) represent one of the most promising, yet most elusive, classes of π-conjugated molecules. [n]Helicenes, that is, ortho-fused helical PAHs, where n is the number of fused benzene rings, endow characteristic chiroptical properties and dynamic behavior owing to their helical chirality. Although enormous progress has been made in the chemistry of helicenes, there are still numerous challenges, including:
The main objective of the proposed research project is to develop synthetic methodology that leads to heretofore unknown, polycyclic, helical, highly polarized molecules possessing pyrrolo[3,2-b]pyrrole and diketopyrrolopyrrole as core structural components. It is envisioned that long/multiple, strongly polarized helicenes of this type will possess the previously unobtainable photophysical properties mentioned above such as very high fluorescence quantum yields and deep-red and NIR emission.
The strategy to achieve these ambitious objectives will be based on a combination of the following:
The pivotal challenges are related to:
Preliminary results reveal it is possible to synthesize double helicenes built on a pyrrolo[3,2-b]pyrrole core via two different strategies. In particular we have shown that double intramolecular direct arylation followed by photochemical electrocyclization is a viable strategy to prepare [6]helicenes.
The results from this project will set new paradigms for both synthetic strategies leading to complex helical structures as well as for fine-tuning critical physicochemical parameters. The long-term impact on the scientific community encompasses the deepened understanding of the relationship between the structure of polarized curved molecules and photophysical properties in general, as well as symmetry-breaking in both the ground and excited states. Our far-reaching goal is to make a significant advancement towards the preparation of chiral Organic Light-Emitting Diodes and Organic Light-Emissive Transistors based on these new helical molecules.
Kielesiński, Ł.; Deperasińska, I.; Morawski, O.; Vygranenko, K. V.; Ouellette, E. T.; Gryko, D. T. ‘Polarized, V-shaped, Conjoined Bis-coumarins: From Lack of Dipole Moment Alignment to High Brightness’ J. Org. Chem. 2022, 87, 5961-5975
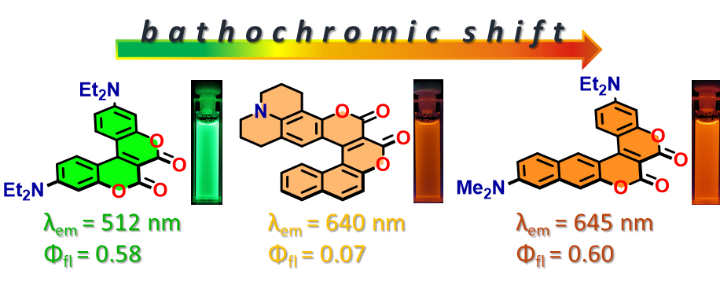
Eleven conjoined coumarins possessing a chromeno[3,4-c]chromene-6,7-dione skeleton have been synthesized via the reaction of electron-rich phenols with esters of coumarin-3-carboxylic acids, catalyzed either by Lewis acids or 4-dimethylaminopyridine. Furthermore, Michael-type addition to angular benzo[f]coumarins is possible, leading to conjugated helical systems. Arrangement of the electron-donating amino groups at diverse positions on this heterocyclic skeleton makes it possible to obtain π-expanded coumarins with emission either sensitive to, or entirely independent of, solvent polarity with large Stokes shifts. Computational studies have provided rationale for moderate solvatochromic effects unveiling lack of collinearity of the dipole moments in the ground and excited states. Depending on the functional groups present, the obtained dyes are highly polarized with dipole moments of ~14 D in the ground and ~20-25 D in the excited state. Strong emission in non-polar solvents, in spite of the inclusion of a NO2 group is rationalized by the fact that the intramolecular charge transfer introduced to these molecules is strong enough to suppress ISC, yet weak enough to prevent formation of dark TICT states. Photochemical transformation of the dye possessing a chromeno[3,4-c]pyridine-4,5-dione scaffold led to the formation of a spirocyclic benzo[g]coumarin.
Members: