Research Topics:
Our interests currently lie in the multi-disciplinary field of supramolecular chemistry. We concentrate on molecular recognition with special emphasis on chiral recognition. We design macromolecular building blocks and synthesize them. We explore the conformational, electronic, recognition and assembly properties of our compounds using a wide range of spectroscopic and analytical techniques. Our current research topics include:
1. Inherent chirality of molecules
With the growing complication of molecular systems that are characterized by chemists, the classical understanding of molecular chirality is not always sufficient. For instance, chirality elements like centre, axis or plane are not adequate to describe the chirality of rotaxanes, catenanes, fullerenes, cavitands or capsular assemblies. Definition of inherent chirality underlines the crucial role of curvature that clearly distinguishes the faces of the object. It was formulated in 2004 by the group of Mandolini and Schiaffino and then re-formulated in our group. The definition currently reads ''inherent chirality arises from the introduction of a curvature in an ideal planar structure that is devoid of perpendicular symmetry planes in its bidimensional representation". In the past years we syntesized and characterized many types of cavitands that exhibit inherent chirality. They were covalent compounds (Fig. 1b) or inherent chirality was the results of non-covalent direcational
interactions (Fig. 1c).

Fig. 1. Objects and molecules that are inherently chiral: a) macroscopic object; b) cavitand with inherent chirality originating from covalent connections; b) inherent chirality by non-covalent arrangement.
2. Assembly of capsules and cavitands with functionalized interiors
Most of the currently known synthetic container molecules (capsules and cavitands) have walls composed of aromatic rings. Therefore, they have smooth and hydrophobic interiors incapable of directional interactions with guests, recognition of polar guests, distinguishing of enantiomers, performing in asymmetric reactions or generation of unidirectional motion. Recently, we designed and synthesized well-ordered capsular dimer with hydrophobic outer surface and polar and chiral interior (Fig. 2). The internal cavity is composed of alternating charged groups of the amino acids. The supramolecular approach to the synthesis, involving a combination of reversible reactions (heterogeneous), self-assembly and filling the capsule in the reaction medium, proved to be indispensable for the successful preparation. Recognition studies show that of 12 can encapsulate various small polar molecules. The interior of the capsule is also very effcient in chiral recognition (up to 99% de for diols).
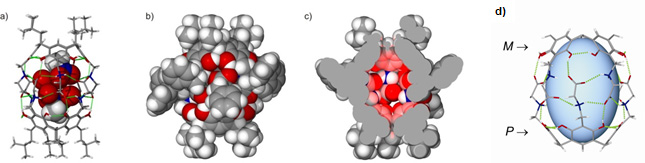
Fig. 2. Chiral reversed molecular capsule: polar inside and hydrophobic outside.
3. Dynamics and reactivity within constrained spaces
The effectiveness of enzymatic catalysis usually comes from a combination of several factors that assure stabilization of a transition state. One of them is bringing the reactants into a close proximity. Additional important factor in enzymatic catalysis is eletrostatic stabilization of polar transition states. The great advantage of an artificial molecular vessel as a reaction medium is separation of reacting species from the hostile environment and bringing reactants into a close proximity. Unfortunatelly, the currently known molecular vessels with hydrophobic cavities have limited possibility of utilizing electrostatic interaction. The capsules designed in our group have interiors that are polar and chiral. Therefore, we expect their better performance in reactions proceeding through polar transition states (majority of the organic reactions) and possible application in asymmetric reactions. Directional interactions that are present between hosts and guests are also for controlling internal dynamics in chiral environment.

Fig. 3. Reactivity and dynamics in constrained spaces.

4. Project POMOST "Novel Chiral Nanospaces for Molecular Confinement"
The goal of this project to design and synthesize new chiral capsules and cavitands having polar and functional interiors with the aim of using them as molecular reactors. The field of molecular containers is already quite devloped, however there is still a very limitted number of known molecular containers with internal functionalities. 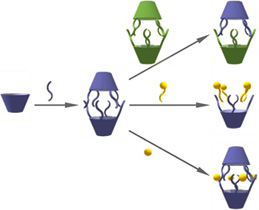 Most of them are hydrophobic inside. Their nonpolar interiors are disadvantageous for catalytic purposes since the only possible catalytic mechanism involves bringing the reactants together, whereas electrostatic effects were identified to have main catalytic contribution for natural systems. Synthetic container molecules with chiral and polar walls capable of directional interactions with guests can potentially overcome these drawbacks and therefore are of great interest. Tasks in the project will involve the synthesis of new polar capsules and cavitands based on amino acids and peptides. The synthesis will include a combination of classical organic synthesis and non-covalent assembly. The design includes capsules having various cavity sizes (in order to accommodate various/multiple guests), chiral cavitand molecules (in order to tune kinetics of guest uptake/release) and metal-organic capsules (in order to modulate properties of capsule's walls. The imortant topic in the project is structural characterization of the assemblies involving molecular modeling, advanced NMR (2D techniques including DOSY), CD and X-ray techniques.
Most of them are hydrophobic inside. Their nonpolar interiors are disadvantageous for catalytic purposes since the only possible catalytic mechanism involves bringing the reactants together, whereas electrostatic effects were identified to have main catalytic contribution for natural systems. Synthetic container molecules with chiral and polar walls capable of directional interactions with guests can potentially overcome these drawbacks and therefore are of great interest. Tasks in the project will involve the synthesis of new polar capsules and cavitands based on amino acids and peptides. The synthesis will include a combination of classical organic synthesis and non-covalent assembly. The design includes capsules having various cavity sizes (in order to accommodate various/multiple guests), chiral cavitand molecules (in order to tune kinetics of guest uptake/release) and metal-organic capsules (in order to modulate properties of capsule's walls. The imortant topic in the project is structural characterization of the assemblies involving molecular modeling, advanced NMR (2D techniques including DOSY), CD and X-ray techniques.
5. Hybrid macrocyclic [n]arenes as new supramolecular scaffolds
Non-covalent interactions are crucial for the existence the whole living world. They govern the enzyme-substrate interactions, formation of double helices, interactions with receptors, signal transmission and many other biological processes. As chemists, we try to mimic the Nature and also create non-covalent assemblies in a controllable way. What is more, we try to make completely new artificial systems for new applications, for example sensing, storage or catalysis. We are still far away behind Nature in terms of effectiveness. However, the field of supramolecular chemistry, that deals with non-covalent assemblies is now quite mature. A lot is already known, since thousands of artificial sensors, storage materials or catalysts have already been tested. Some general strategies have been developed by now. For the construction of effective supramolecular sensors a generally accepted strategy is to utilize semi-rigid and semi-open molecules as scaffolds and further modify them to get desirable properties. Such approach has some advantages: a) upon substrate binding the semi-rigid structure allows for small error-corrections and, on the other hand, does not have a huge entropic penalty of a fully labile receptor; b) semi-open cavity is preferable due to both entropic (already open, no rearrangement penalty, solvent release benefits) and enthalpic reasons (possibility of placement of binding sites in a convergent way). Therefore, the synthesis of semi-rigid macrocyclic platforms is fundamental for further receptor design. There are several well-known and highly used macrocylic scaffolds including: calix[n]arenes, resorcin[4]arenes, cyclotriveratrylenes, and cucurbit[n]urils. There are many interesting applications using these scaffolds including sensing, storage of gases and reactive intermediates, construction of large nanocontainers and catalytic reaction nanovessels. However, the shapes of macrocylic skeletons limit their applications to some extent. It is still an important goal to obtain new semi-rigid macrocycles that may serve as new supramolecular scaffolds with new shapes and/or new binding properties.
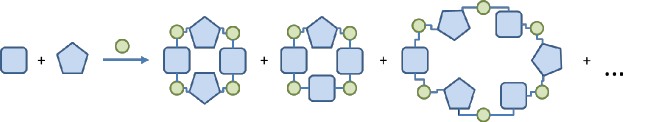
Fig. 5. The schematic representation of the synthesis of hybrid macrocycles.
The aim of the current project is to obtain new macrocylic compounds composed of poliphenolic units (Fig. 5). We plan to use various known polyphenolic building blocks and combine them using the methodology that was recently developed in our group in order to obtain hybrid macrocycles of various shapes using templated or non-templated synthesis. We will determine the structures of the resulting macrocycles and probe their complexing properties towards representative guest molecules. We will also try to functionalize them, in order to check their versatility. The great advantage at this point is that the macrocycles with new shapes will be composed of well-known building blocks. Therefore, their chemistry will be very rich and easy to predict. Finally, we would like to develop an efficient, scalable synthetic methodology for most promising macrocyles in order to make them useful macrocyclic scaffolds for supramolecular chemistry.
